Composition, structure and function of the Helicobacter pylori cag pathogenicity island encoded type IV secretion system
- PMID: 26059619
- PMCID: PMC4493163
- DOI: 10.2217/fmb.15.32
Composition, structure and function of the Helicobacter pylori cag pathogenicity island encoded type IV secretion system
Abstract
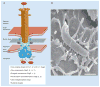
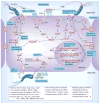
Many Gram-negative pathogens harbor type IV secretion systems (T4SS) that translocate bacterial virulence factors into host cells to hijack cellular processes. The pathology of the gastric pathogen Helicobacter pylori strongly depends on a T4SS encoded by the cag pathogenicity island. This T4SS forms a needle-like pilus, and its assembly is accomplished by multiple protein-protein interactions and various pilus-associated factors that bind to integrins followed by delivery of the CagA oncoprotein into gastric epithelial cells. Recent studies revealed the crystal structures of six T4SS proteins and pilus formation is modulated by iron and zinc availability. All these T4SS interactions are crucial for deregulating host signaling events and disease progression. New developments in T4SS functions and their importance for pathogenesis are discussed.
Keywords: CagA; CagL; CagY; EGF receptor; GTPase; Helicobacter pylori; MARK2/PAR1b kinase; T4SS; VirB proteins; c-Met; cagPAI; cortactin; gastric disease; integrin; signaling; type IV secretion; tyrosine kinases; β-catenin.
Figures
References
-
- Gal-Mor O, Finlay BB. Pathogenicity islands: a molecular toolbox for bacterial virulence. Cell Microbiol. 2006;8(11):1707–1719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
