Less Bone Loss With Maraviroc- Versus Tenofovir-Containing Antiretroviral Therapy in the AIDS Clinical Trials Group A5303 Study
- PMID: 26060295
- PMCID: PMC4560904
- DOI: 10.1093/cid/civ455
Less Bone Loss With Maraviroc- Versus Tenofovir-Containing Antiretroviral Therapy in the AIDS Clinical Trials Group A5303 Study
Abstract
Background: There is a need to prevent or minimize bone loss associated with antiretroviral treatment (ART) initiation. We compared maraviroc (MVC)- to tenofovir disoproxil fumarate (TDF)-containing ART.
Methods: This was a double-blind, placebo-controlled trial. ART-naive subjects with human immunodeficiency virus type 1 RNA load (viral load [VL]) >1000 copies/mL and R5 tropism were randomized to MVC 150 mg or TDF 300 mg once daily (1:1), stratified by VL <100 000 or ≥100 000 copies/mL and age <30 or ≥30 years. All subjects received darunavir 800 mg, ritonavir 100 mg, and emtricitabine 200 mg daily. Dual-energy X-ray absorptiometry scanning was done at baseline and week 48. The primary endpoint was percentage change in total hip bone mineral density (BMD) from baseline to week 48 in the as-treated population.
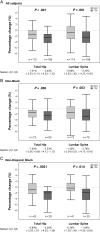
Results: We enrolled 262 subjects. A total of 259 subjects (130 MVC, 129 TDF) contributed to the analyses (91% male; median age, 33 years; 45% white, 30% black, 22% Hispanic). Baseline median VL was 4.5 log10 copies/mL and CD4 count was 390 cells/µL. The decline in hip BMD (n = 115 for MVC, n = 109 for TDF) at week 48 was less with MVC (median [Q1, Q3] of -1.51% [-2.93%, -0.11%] vs -2.40% [-4.30%, -1.32%] for TDF (P < .001). Lumbar spine BMD decline was also less with MVC (median -0.88% vs -2.35%; P < .001). Similar proportions of subjects in both arms achieved VL ≤50 copies/mL in as-treated and ITT analyses.
Conclusions: MVC was associated with less bone loss at the hip and lumbar spine compared with TDF. MVC may be an option to attenuate ART-associated bone loss.
Clinical trials registration: NCT01400412.
Keywords: bone; darunavir; maraviroc; tenofovir.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Duvivier C, Kolta S, Assoumou L et al. . Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 2009; 27:817–24. - PubMed
-
- van Vonderen MG, Lips P, van Agtmael MA et al. . First line zidovudine/lamivudine/ lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS 2009; 23:1367–76. - PubMed
-
- McComsey GA, Kitch D, Daar E et al. . Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011; 203:1791–801. - PMC - PubMed
-
- Brown TT, McComsey GA, King MS et al. . Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009; 51:554–61. - PubMed
-
- Stellbrink HJ, Orkin C, Arribas JR et al. . Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010; 51:963–72. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069503/AI/NIAID NIH HHS/United States
- 2UM1AI069439-08/AI/NIAID NIH HHS/United States
- U01 AI069447/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- UM1 AI069472/AI/NIAID NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- 2UMAI069432/PHS HHS/United States
- UL1 TR001111/TR/NCATS NIH HHS/United States
- U01AI069447/AI/NIAID NIH HHS/United States
- UL1TR000454/TR/NCATS NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- UM1AI069471/AI/NIAID NIH HHS/United States
- 2UM1-AI069470-08/AI/NIAID NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- P30AI073961/AI/NIAID NIH HHS/United States
- 2UM1AI069432/AI/NIAID NIH HHS/United States
- N01-HD-3-3345/HD/NICHD NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- AI069501/AI/NIAID NIH HHS/United States
- AI 69501/AI/NIAID NIH HHS/United States
- 2UM1AI069503/AI/NIAID NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- 1U01AI069477-01/AI/NIAID NIH HHS/United States
- 2UM1AI069412-08/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- UM1A 068636-09/PHS HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- 2P30 AI 50409-10/AI/NIAID NIH HHS/United States
- U01 AI069418/AI/NIAID NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- 2UM1AI069418-08/AI/NIAID NIH HHS/United States
- P30 AI50410/AI/NIAID NIH HHS/United States
- UL1 TR002489/TR/NCATS NIH HHS/United States
- UM1 AI069415/AI/NIAID NIH HHS/United States
- 5UM1AI069415-10/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- 1UL1TR001111/TR/NCATS NIH HHS/United States
- UM1AI069452/AI/NIAID NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- 5UM1 AI068636/AI/NIAID NIH HHS/United States
- AI068636/AI/NIAID NIH HHS/United States
- UMAI069432/PHS HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- P30 AI073961/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- AI069424/AI/NIAID NIH HHS/United States
- 5UM1AI069412/AI/NIAID NIH HHS/United States
- UM1AI 069471/AI/NIAID NIH HHS/United States
- UL1 RR024160/RR/NCRR NIH HHS/United States
- 5 P30 AI-045008-15/AI/NIAID NIH HHS/United States
- AI69439/AI/NIAID NIH HHS/United States
- UL1TR001079/TR/NCATS NIH HHS/United States
- 2UMIA1069423-08/PHS HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- U01 AI069424/AI/NIAID NIH HHS/United States
- UMI AI069511/AI/NIAID NIH HHS/United States
- U01 AI069477/AI/NIAID NIH HHS/United States
- AI068634/AI/NIAID NIH HHS/United States
- UMAI069494/PHS HHS/United States
- UM1AI069472/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- U01AI068636/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

