The VariLift(®) Interbody Fusion System: expandable, standalone interbody fusion
- PMID: 26060414
- PMCID: PMC4454196
- DOI: 10.2147/MDER.S84715
The VariLift(®) Interbody Fusion System: expandable, standalone interbody fusion
Abstract
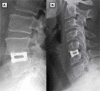
Intervertebral fusion cages have been in clinical use since the 1990s. Cages offer the benefits of bone graft containment, restored intervertebral and foraminal height, and a more repeatable, stable procedure compared to interbody fusion with graft material alone. Due to concerns regarding postoperative stability, loss of lordosis, and subsidence or migration of the implant, interbody cages are commonly used with supplemental fixation such as pedicle screw systems or anterior plates. While providing additional stability, supplemental fixation techniques increase operative time, exposure, cost, and morbidity. The VariLift(®) Interbody Fusion System (VariLift(®) system) has been developed as a standalone solution to provide the benefits of intervertebral fusion cages without the requirement of supplemental fixation. The VariLift(®) system, FDA-cleared for standalone use in both the cervical and lumbar spine, is implanted in a minimal profile and then expanded in situ to provide segmental stability, restored lordosis, and a large graft chamber. Preclinical testing and analyses have found that the VariLift(®) System is durable, and reduces stresses that may contribute to subsidence and migration of other standalone interbody cages. Fifteen years of clinical development with the VariLift(®) system have demonstrated positive clinical outcomes, continued patient maintenance of segmental stability and lordosis, and no evidence of implant migration. The purpose of this report is to describe the VariLift(®) system, including implant characteristics, principles of operation, indications for use, patient selection criteria, surgical technique, postoperative care, preclinical testing, and clinical experience. The VariLift(®) System represents an improved surgical option for a stable interbody fusion without requiring supplemental fixation.
Keywords: ACDF; PLIF; TLIF; standalone cage.
Figures






References
-
- Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10(2):154–168. - PubMed
-
- Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15(6):602–617. - PubMed
-
- Bagby GW. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics. 1988;11(6):931–934. - PubMed
-
- FDA . Premarket Approval of BAK™ Interbody Fusion System with Instrumentation. Silver Spring; United States Food and Drug Administration: 1996. p. 950002.
-
- Kuslich SD, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD. The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2-year follow-up results of a United States prospective, multicenter trial. Spine (Phila Pa 1976) 1998;23(11):1267–1278. discussion 1279. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

