Membrainy: a 'smart', unified membrane analysis tool
- PMID: 26060507
- PMCID: PMC4460882
- DOI: 10.1186/s13029-015-0033-7
Membrainy: a 'smart', unified membrane analysis tool
Abstract
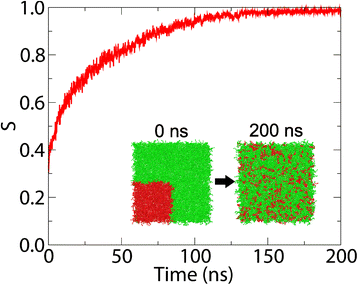
Background: The study of biological membranes using Molecular Dynamics has become an increasingly popular means by which to investigate the interactions of proteins, peptides and potentials with lipid bilayers. These interactions often result in changes to the properties of the lipids which can modify the behaviour of the membrane. Membrainy is a unified membrane analysis tool that contains a broad spectrum of analytical techniques to enable: measurement of acyl chain order parameters; presentation of 2D surface and thickness maps; determination of lateral and axial headgroup orientations; measurement of bilayer and leaflet thickness; analysis of the annular shell surrounding membrane-embedded objects; quantification of gel percentage; time evolution of the transmembrane voltage; area per lipid calculations; and quantification of lipid mixing/demixing entropy.
Results: Each analytical component within Membrainy has been tested on a variety of lipid bilayer systems and was found to be either comparable to or an improvement upon existing software. For the analytical techniques that have no direct comparable software, our results were confirmed with experimental data.
Conclusions: Membrainy is a user-friendly, intelligent membrane analysis tool that automatically interprets a variety of input formats and force fields, is compatible with both single and double bilayers, and capable of handling asymmetric bilayers and lipid flip-flopping. Membrainy has been designed for ease of use, requiring no installation or configuration and minimal user-input to operate.
Keywords: Area per lipid; Asymmetric bilayer; Bilayer/Leaflet thickness; Double bilayer; Headgroup orientations; Lipid flip-flopping; Membrane analysis; Mixing/Demixing entropy; Molecular dynamics; Order parameters.
Figures




References
-
- Penkett S, Flook A, Chapman D. Physical studies of phospholipids. ix. nuclear resonance studies of lipid-water systems. Chem Phys Lipids. 1968;2:273–90. doi: 10.1016/0009-3084(68)90004-2. - DOI
-
- Schindler H, Seelig J. Epr spectra of spin labels in lipid bilayers. J Chem Phys. 1973;59:1841–50. doi: 10.1063/1.1680269. - DOI
-
- De Kruijff B, Verkley A, Van Echteld C, Gerritsen W, Mombers C, Noordam P, et al. The occurrence of lipidic particles in lipid bilayers as seen by 31P NMR and freeze-fracture electron-microscopy. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1979;555:200–9. doi: 10.1016/0005-2736(79)90160-3. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

