Safety and Efficacy of Bimatoprost for Eyelash Growth in Postchemotherapy Subjects
- PMID: 26060513
- PMCID: PMC4456802
Safety and Efficacy of Bimatoprost for Eyelash Growth in Postchemotherapy Subjects
Abstract
Objective: To evaluate long-term efficacy and safety of bimatoprost for treatment of chemotherapy-induced eyelash hypotrichosis.
Design: One-year, multicenter, double-masked, parallel-group study.
Setting: Twenty-one centers in the United States and one center in the United Kingdom.
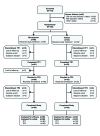
Participants: This study randomized (3:1) 130 subjects to bimatoprost 0.03% or vehicle applied topically to upper eyelid margins for six months. All subjects used bimatoprost for a second six months.
Measurements: Responders for the primary composite end point achieved ≥1-grade improvement in Global Eyelash Assessment score and ≥3-point improvement in Confidence, Attractiveness, and Professionalism domain score of the Eyelash Satisfaction Questionnaire at Month 4. Secondary assessments included eyelash length, thickness, and darkness, using digital image analysis.
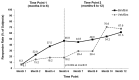
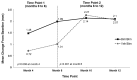
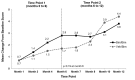
Results: The responder rate was significantly higher with bimatoprost versus vehicle at Month 4 (37.5% vs. 18.2%; p=0.041) and Month 6 (46.9% vs. 18.2%; p=0.004). Significant improvements favoring bimatoprost occurred in eyelash length (p=0.008), thickness (p<0.001), or darkness (p=0.029) at Month 4, with similar results at Month 6 (p<0.001, length; p<0.001, thickness; p=0.002, darkness). Responder rates reached 61.5 percent at Month 12 for subjects continuing bimatoprost and 67.6 percent for those switched from vehicle to bimatoprost. Conjunctival hyperemia (16.7%) and punctate keratitis (9.4%) were the most common adverse events.
Conclusion: Bimatoprost provides rapid eyelash recovery, whether started shortly after chemotherapy (4 to 12 weeks) or delayed for six months, with minimal adverse events.
Clinical trial registry: NCT00907426.
Figures










References
-
- Shaikh MY, Bodla AA. Hypertrichosis of the eyelashes from prostaglandin analog use: a blessing or a bother to the patient? J Ocul Pharmacol Ther. 2006;22:76–77. - PubMed
-
- Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17:317–328. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
