Selective stabilization of aliphatic organic carbon by iron oxide
- PMID: 26061259
- PMCID: PMC4462107
- DOI: 10.1038/srep11214
Selective stabilization of aliphatic organic carbon by iron oxide
Abstract
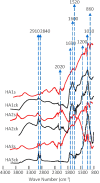
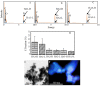
Stabilization of organic matter in soil is important for natural ecosystem to sequestrate carbon and mitigate greenhouse gas emission. It is largely unknown what factors govern the preservation of organic carbon in soil, casting shadow on predicting the response of soil to climate change. Iron oxide was suggested as an important mineral preserving soil organic carbon. However, ferric minerals are subject to reduction, potentially releasing iron and decreasing the stability of iron-bound organic carbon. Information about the stability of iron-bound organic carbon in the redox reaction is limited. Herein, we investigated the sorptive interactions of organic matter with hematite and reductive release of hematite-bound organic matter. Impacts of organic matter composition and conformation on its sorption by hematite and release during the reduction reaction were analyzed. We found that hematite-bound aliphatic carbon was more resistant to reduction release, although hematite preferred to sorb more aromatic carbon. Resistance to reductive release represents a new mechanism that aliphatic soil organic matter was stabilized by association with iron oxide. Selective stabilization of aliphatic over aromatic carbon can greatly contribute to the widely observed accumulation of aliphatic carbon in soil, which cannot be explained by sorptive interactions between minerals and organic matter.
Figures
References
-
- Amundson R. The carbon budget in soils. Annu. Rev. Earth Pl. Sc. 29, 535–562 (2001).
-
- Houghton R. A. Balancing the global carbon budget. Annu. Rev. Earth Pl. Sc. 35, 313–347 (2007).
-
- Lal R. Offsetting global CO2 emissions by restoration of degraded soils and intensification of world agriculture and forestry. Land Degrad. Dev. 14, 309–322 (2003).
-
- Lal R. Soil carbon sequestration impacts on global climate change and food security. Science 304, 1623–1627 (2004). - PubMed
-
- Li J., Ziegler S. E., Lane C. S. & Billings S. A. Legacies of native climate regime govern responses of boreal soil microbes to litter stoichiometry and temperature. Soil Biol. Biochem. 66, 204–213 (2013).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

