Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx
- PMID: 26061344
- PMCID: PMC4744710
- DOI: 10.1002/mrd.22500
Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx
Abstract
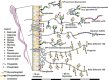
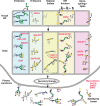
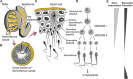
Mammalian spermatozoa are coated with a thick glycocalyx that is assembled during sperm development, maturation, and upon contact with seminal fluid. The sperm glycocalyx is critical for sperm survival in the female reproductive tract and is modified during capacitation. The complex interplay among the various glycoconjugates generates numerous signaling motifs that may regulate sperm function and, as a result, fertility. Nascent spermatozoa assemble their own glycans while the cells still possess a functional endoplasmic reticulum and Golgi in the seminiferous tubule, but once spermatogenesis is complete, they lose the capacity to produce glycoconjugates de novo. Sperm glycans continue to be modified, during epididymal transit by extracellular glycosidases and glycosyltransferases. Furthermore, epididymal cells secrete glycoconjugates (glycophosphatidylinositol-anchored glycoproteins and glycolipids) and glycan-rich microvesicles that can fuse with the maturing sperm membrane. The sperm glycocalyx mediates numerous functions in the female reproductive tract, including the following: inhibition of premature capacitation; passage through the cervical mucus; protection from innate and adaptive female immunity; formation of the sperm reservoir; and masking sperm proteins involved in fertilization. The immense diversity in sperm-associated glycans within and between species forms a remarkable challenge to our understanding of essential sperm glycan functions.
© 2015 The Authors. Molecular Reproduction and Development published by Wiley Periodicals, Inc.
Figures

References
-
- Akama TO, Nakagawa H, Sugihara K, Narisawa S, Ohyama C, Nishimura S, O'Brien DA, Moremen KW, Millan JL, Fukuda MN. 2002. Germ cell survival through carbohydrate‐mediated interaction with Sertoli cells. Science 295:124‐ 127. - PubMed
-
- Alghamdi AS, Foster DN. 2005. Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol Reprod 73:1174–1181. - PubMed
-
- Andersch‐Bjorkman Y, Thomsson KA, Holmen Larsson JM, Ekerhovd E, Hansson GC. 2007. Large scale identification of proteins, mucins, and their O‐glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics 6:708–716. - PubMed
-
- Arenas MI, de Miguel MP, Bethencourt FR, Fraile B, Royuela M, Paniagua R. 1996. Lectin histochemistry in the human epididymis. J Reprod Fertil 106:313–320. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

