Macrophage-derived tumor necrosis factor-α mediates diabetic renal injury
- PMID: 26061548
- PMCID: PMC4589442
- DOI: 10.1038/ki.2015.162
Macrophage-derived tumor necrosis factor-α mediates diabetic renal injury
Abstract
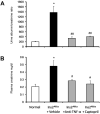
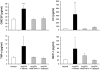
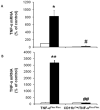
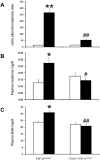
Monocyte/macrophage recruitment correlates strongly with the progression of diabetic nephropathy. Tumor necrosis factor-α (TNF-α) is produced by monocytes/macrophages but the direct role of TNF-α and/or macrophage-derived TNF-α in the progression of diabetic nephropathy remains unclear. Here we tested whether inhibition of TNF-α confers kidney protection in diabetic nephropathy via a macrophage-derived TNF-α-dependent pathway. Compared to vehicle-treated mice, blockade of TNF-α with a murine anti-TNF-α antibody conferred kidney protection in Ins2(Akita) mice as indicated by reductions in albuminuria, plasma creatinine, histopathologic changes, kidney macrophage recruitment, and plasma inflammatory cytokine levels at 18 weeks of age. To assess the direct role of macrophage-derived TNF-α in diabetic nephropathy, we generated macrophage-specific TNF-α-deficient mice (CD11b(Cre)/TNF-α(Flox/Flox)). Conditional ablation of TNF-α in macrophages significantly reduced albuminuria, the increase in plasma creatinine and blood urea nitrogen, histopathologic changes, and kidney macrophage recruitment compared to diabetic TNF-α(Flox/Flox) control mice after 12 weeks of streptozotocin-induced diabetes. Thus, production of TNF-α by macrophages plays a major role in diabetic renal injury. Hence, blocking TNF-α could be a novel therapeutic approach for treatment of diabetic nephropathy.
Figures




Comment in
-
Relevance of TNF-α in the context of other inflammatory cytokines in the progression of diabetic nephropathy.Kidney Int. 2015 Oct;88(4):662-5. doi: 10.1038/ki.2015.250. Kidney Int. 2015. PMID: 26422621 Free PMC article.
References
-
- Baud L, Oudinet JP, Bens M, et al. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989;35:1111–1118. - PubMed
-
- Hruby ZW, Lowry RP. Spontaneous release of tumor necrosis factor alpha by isolated renal glomeruli and cultured glomerular mesangial cells. Clin Immunol Immunopathol. 1991;59:156–164. - PubMed
-
- Tipping PG, Leong TW, Holdsworth SR. Tumor necrosis factor production by glomerular macrophages in anti-glomerular basement membrane glomerulonephritis in rabbits. Lab Invest. 1991;65:272–279. - PubMed
-
- Wu TH, Wu SC, Huang TP, et al. Increased excretion of tumor necrosis factor alpha and interleukin 1 beta in urine from patients with IgA nephropathy and Schonlein-Henoch purpura. Nephron. 1996;74:79–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

