Cortical Thickness in Dementia with Lewy Bodies and Alzheimer's Disease: A Comparison of Prodromal and Dementia Stages
- PMID: 26061655
- PMCID: PMC4489516
- DOI: 10.1371/journal.pone.0127396
Cortical Thickness in Dementia with Lewy Bodies and Alzheimer's Disease: A Comparison of Prodromal and Dementia Stages
Abstract
Objectives: To assess and compare cortical thickness (CTh) of patients with prodromal Dementia with Lewy bodies (pro-DLB), prodromal Alzheimer's disease (pro-AD), DLB dementia (DLB-d), AD dementia (AD-d) and normal ageing.
Methods: Study participants(28 pro-DLB, 27 pro-AD, 31 DLB-d, 54 AD-d and 33 elderly controls) underwent 3Tesla T1 3D MRI and detailed clinical and cognitive assessments. We used FreeSurfer analysis package to measure CTh and investigate patterns of cortical thinning across groups.
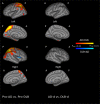
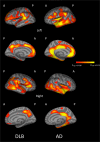
Results: Comparison of CTh between pro-DLB and pro-AD (p<0.05, FDR corrected) showed more right anterior insula thinning in pro-DLB, and more bilateral parietal lobe and left parahippocampal gyri thinning in pro-AD. Comparison of prodromal patients to healthy elderly controls showed the involvement of the same regions. In DLB-d (p<0.05, FDR corrected) cortical thinning was found predominantly in the right temporo-parietal junction, and insula, cingulate, orbitofrontal and lateral occipital cortices. In AD-d(p<0.05, FDR corrected),the most significant areas affected included the entorhinal cortices, parahippocampal gyri and parietal lobes. The comparison of AD-d and DLB-d demonstrated more CTh in AD-d in the left entorhinal cortex (p<0.05, FDR corrected).
Conclusion: Cortical thickness is a sensitive measure for characterising patterns of grey matter atrophy in early stages of DLB distinct from AD. Right anterior insula involvement may be a key region at the prodromal stage of DLB and needs further investigation.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

