A randomized controlled trial on the effects of goal-directed therapy on the inflammatory response open abdominal aortic aneurysm repair
- PMID: 26062689
- PMCID: PMC4479246
- DOI: 10.1186/s13054-015-0974-x
A randomized controlled trial on the effects of goal-directed therapy on the inflammatory response open abdominal aortic aneurysm repair
Abstract
Introduction: Goal-directed therapy (GDT) has been shown in numerous studies to decrease perioperative morbidity and mortality. The mechanism of benefit of GDT, however, has not been clearly elucidated. Targeted resuscitation of the vascular endothelium with GDT might alter the postoperative inflammatory response and be responsible for the decreased complications with this therapy.
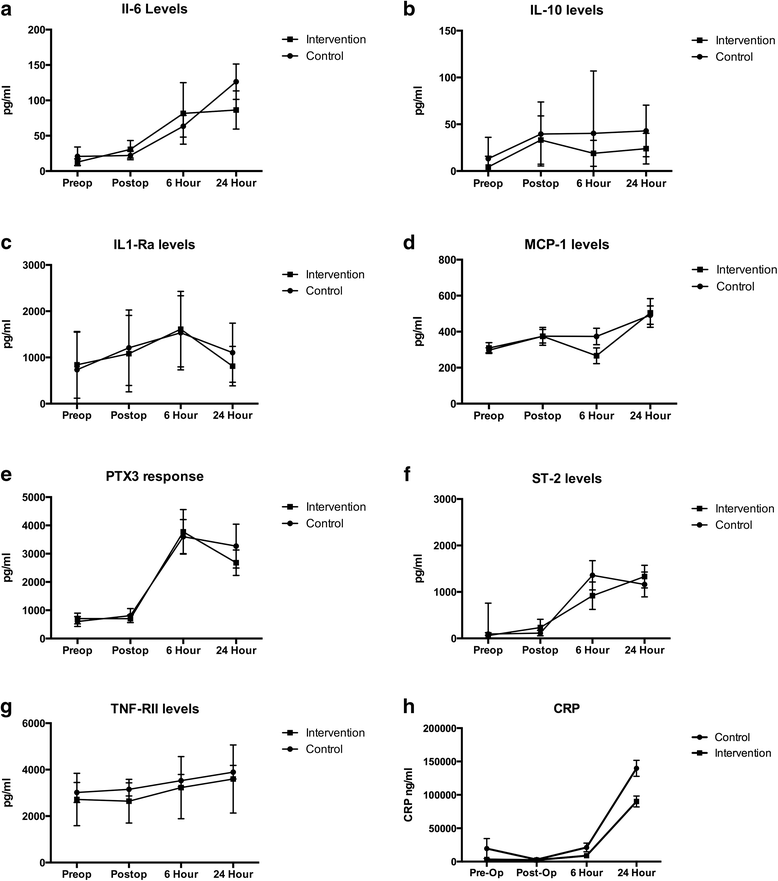
Methods: This trial was registered at ClinicalTrials.gov as NCT01681251. Forty patients undergoing elective open repair of their abdominal aortic aneurysm, 18 years of age and older, were randomized to an interventional arm with GDT targeting stroke volume variation with an arterial pulse contour cardiac output monitor, or control, where fluid therapy was administered at the discretion of the attending anesthesiologist. We measured levels of several inflammatory cytokines (C-reactive protein, Pentraxin 3, suppressor of tumorgenicity--2, interleukin-1 receptor antagonist, and tumor necrosis factor receptor-III) preoperatively and at several postoperative time points to determine if there was a difference in inflammatory response. We also assessed each group for a composite of postoperative complications.
Results: Twenty patients were randomized to GDT and twenty were randomized to control. Length of stay was not different between groups. Intervention patients received less crystalloid and more colloid. At the end of the study, intervention patients had a higher cardiac index (3.4 ± 0.5 vs. 2.5 ± 0.7 l/minute per m(2), p < 0.01) and stroke volume index (50.1 ± 7.4 vs. 38.1 ± 9.8 ml/m(2), p < 0.01) than controls. There were significantly fewer complications in the intervention than control group (28 vs. 12, p = 0.02). The length of hospital and ICU stay did not differ between groups. There was no difference in the levels of inflammatory cytokines between groups.
Conclusions: Despite being associated with fewer complications and improved hemodynamics, there was no difference in the inflammatory response of patients treated with GDT. This suggests that the clinical benefit of GDT occurs in spite of a similar inflammatory burden. Further work needs to be performed to delineate the mechanism of benefit of GDT.
Trial registration: ClinicalTrials.gov Identifier: NCT01681251 . Registered 18 May 2011.
Figures
References
-
- Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–8. doi: 10.1097/01.sla.0000094387.50865.23. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

