Independent genomewide screens identify the tumor suppressor VTRNA2-1 as a human epiallele responsive to periconceptional environment
- PMID: 26062908
- PMCID: PMC4464629
- DOI: 10.1186/s13059-015-0660-y
Independent genomewide screens identify the tumor suppressor VTRNA2-1 as a human epiallele responsive to periconceptional environment
Abstract
Background: Interindividual epigenetic variation that occurs systemically must be established prior to gastrulation in the very early embryo and, because it is systemic, can be assessed in easily biopsiable tissues. We employ two independent genome-wide approaches to search for such variants.
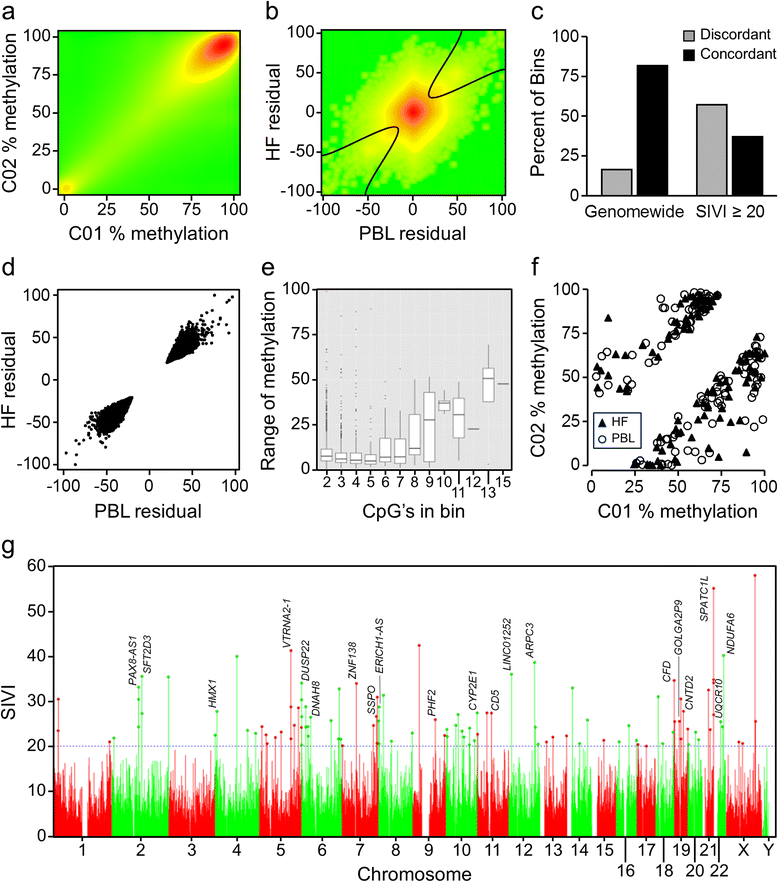
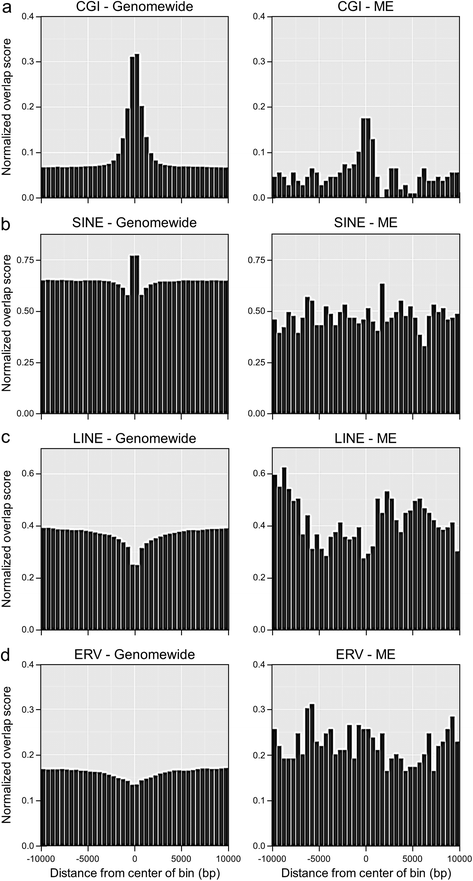
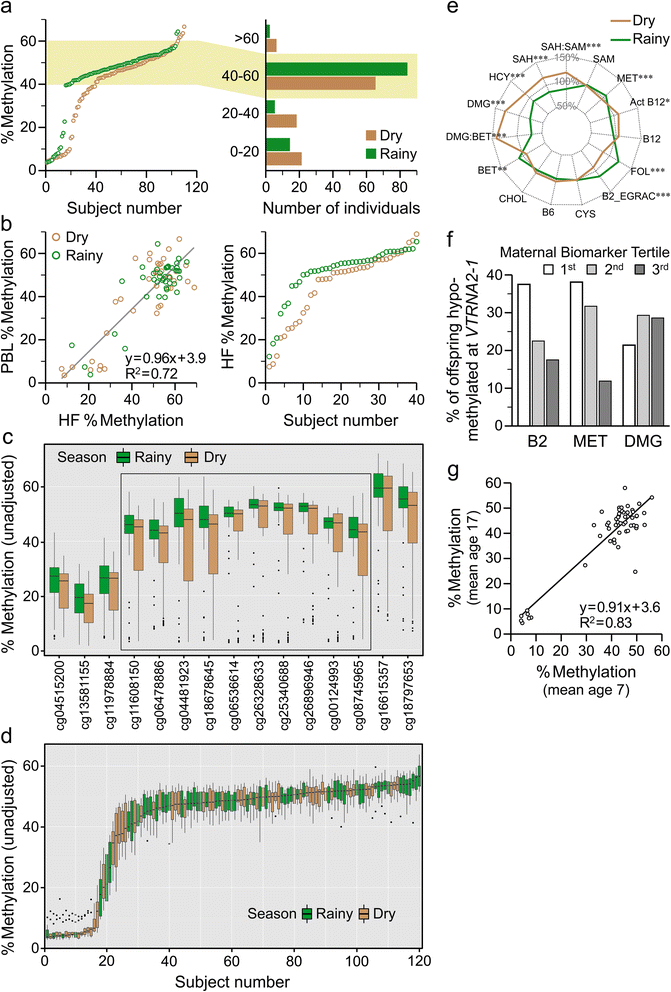
Results: First, we screen for metastable epialleles by performing genomewide bisulfite sequencing in peripheral blood lymphocyte (PBL) and hair follicle DNA from two Caucasian adults. Second, we conduct a genomewide screen for genomic regions at which PBL DNA methylation is affected by season of conception in rural Gambia. Remarkably, both approaches identify the genomically imprinted VTRNA2-1 as a top environmentally responsive epiallele. We demonstrate systemic and stochastic interindividual variation in DNA methylation at the VTRNA2-1 differentially methylated region in healthy Caucasian and Asian adults and show, in rural Gambians, that periconceptional environment affects offspring VTRNA2-1 epigenotype, which is stable over at least 10 years. This unbiased screen also identifies over 100 additional candidate metastable epialleles, and shows that these are associated with cis genomic features including transposable elements.
Conclusions: The non-coding VTRNA2-1 transcript (also called nc886) is a putative tumor suppressor and modulator of innate immunity. Thus, these data indicating environmentally induced loss of imprinting at VTRNA2-1 constitute a plausible causal pathway linking early embryonic environment, epigenetic alteration, and human disease. More broadly, the list of candidate metastable epialleles provides a resource for future studies of epigenetic variation and human disease.
Figures

References
-
- Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012;13:97–109. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

