Investigating the contribution of IL-17A and IL-17F to the host response during Escherichia coli mastitis
- PMID: 26062913
- PMCID: PMC4462179
- DOI: 10.1186/s13567-015-0201-4
Investigating the contribution of IL-17A and IL-17F to the host response during Escherichia coli mastitis
Abstract
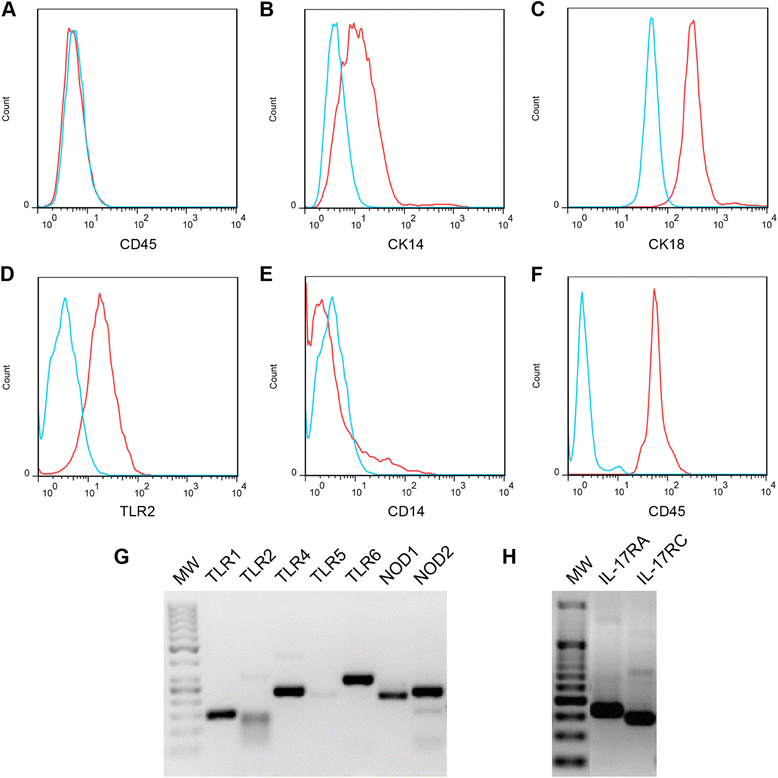
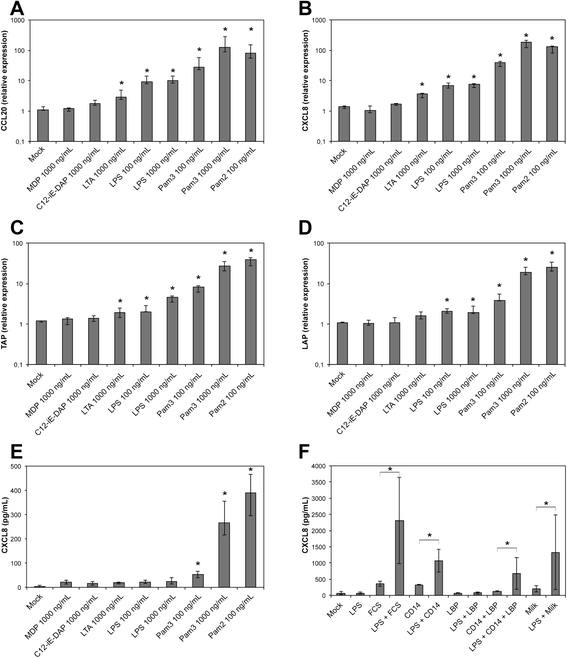
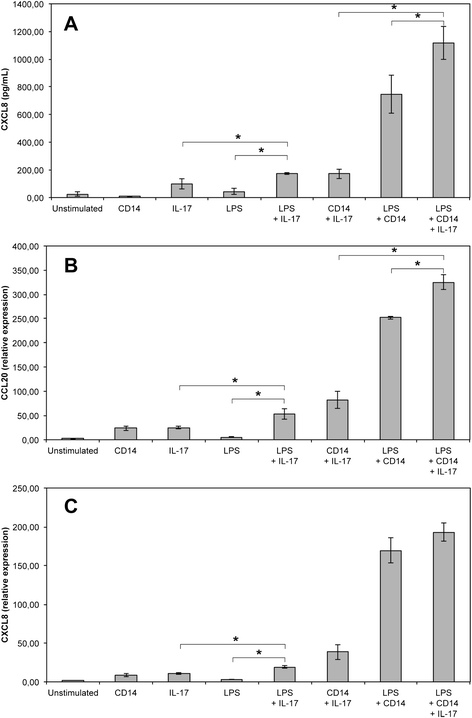
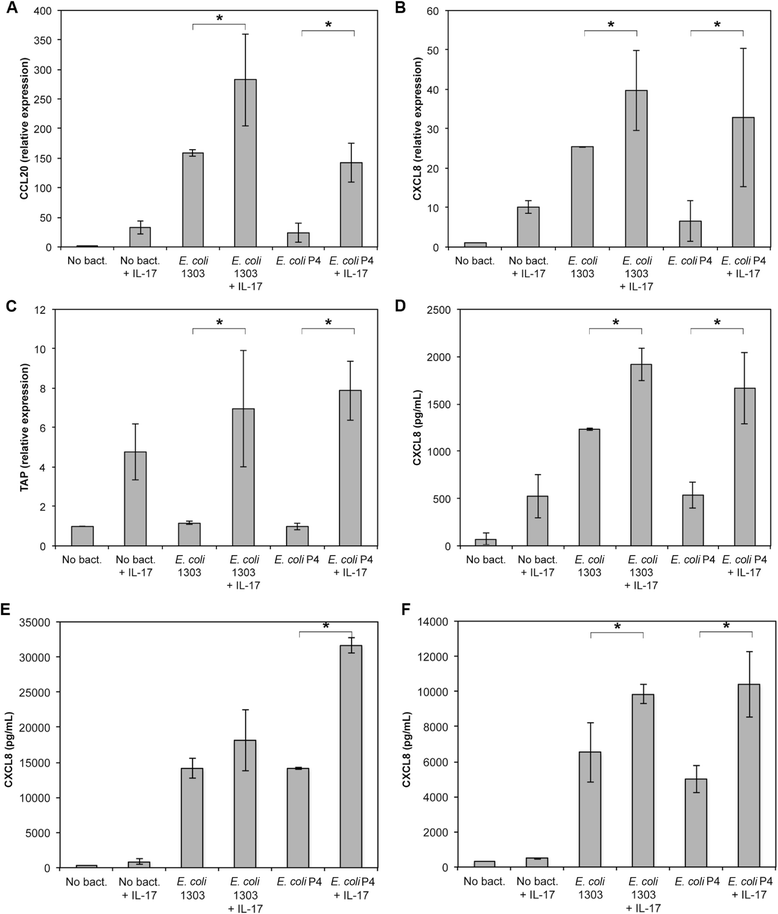
Mastitis remains a major disease of cattle with a strong impact on the dairy industry. There is a growing interest in understanding how cell mediated immunity contributes to the defence of the mammary gland against invading mastitis causing bacteria. Cytokines belonging to the IL-17 family, and the cells that produce them, have been described as important modulators of the innate immunity, in particular that of epithelial cells. We report here that expression of IL-17A and IL-17F genes, encoding two members of the IL-17 family, are induced in udder tissues of cows experimentally infected with Escherichia coli. The impact of IL-17A on the innate response of bovine mammary epithelial cells was investigated using a newly isolated cell line, the PS cell line. We first showed that PS cells, similar to primary bovine mammary epithelial cells, were able to respond to agonists of TLR2 and to LPS, provided CD14 was added to the culture medium. We then showed that secretion of CXCL8 and transcription of innate immunity related-genes by PS cells were increased by IL-17A, in particular when these cells were stimulated with live E. coli bacteria. Together with data from the literature, these results support the hypothesis that IL-17A and IL-17 F could play an important role in mediating of host-pathogen interactions during mastitis.
Figures


References
-
- Schukken YH, Gunther J, Fitzpatrick J, Fontaine MC, Goetze L, Holst O, Leigh J, Petzl W, Schuberth HJ, Sipka A, Smith DG, Quesnell R, Watts J, Yancey R, Zerbe H, Gurjar A, Zadoks RN, Seyfert HM. Host-response patterns of intramammary infections in dairy cows. Vet Immunol Immunopathol. 2011;144:270–289. doi: 10.1016/j.vetimm.2011.08.022. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

