Prenatal Iron Supplementation Reduces Maternal Anemia, Iron Deficiency, and Iron Deficiency Anemia in a Randomized Clinical Trial in Rural China, but Iron Deficiency Remains Widespread in Mothers and Neonates
- PMID: 26063068
- PMCID: PMC4516762
- DOI: 10.3945/jn.114.208678
Prenatal Iron Supplementation Reduces Maternal Anemia, Iron Deficiency, and Iron Deficiency Anemia in a Randomized Clinical Trial in Rural China, but Iron Deficiency Remains Widespread in Mothers and Neonates
Abstract
Background: Previous trials of prenatal iron supplementation had limited measures of maternal or neonatal iron status.
Objective: The purpose was to assess effects of prenatal iron-folate supplementation on maternal and neonatal iron status.
Methods: Enrollment occurred June 2009 through December 2011 in Hebei, China. Women with uncomplicated singleton pregnancies at ≤20 wk gestation, aged ≥18 y, and with hemoglobin ≥100 g/L were randomly assigned 1:1 to receive daily iron (300 mg ferrous sulfate) or placebo + 0.40 mg folate from enrollment to birth. Iron status was assessed in maternal venous blood (at enrollment and at or near term) and cord blood. Primary outcomes were as follows: 1) maternal iron deficiency (ID) defined in 2 ways as serum ferritin (SF) <15 μg/L and body iron (BI) <0 mg/kg; 2) maternal ID anemia [ID + anemia (IDA); hemoglobin <110 g/L]; and 3) neonatal ID (cord blood ferritin <75 μg/L or zinc protoporphyrin/heme >118 μmol/mol).
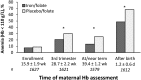
Results: A total of 2371 women were randomly assigned, with outcomes for 1632 women or neonates (809 placebo/folate, 823 iron/folate; 1579 mother-newborn pairs, 37 mothers, 16 neonates). Most infants (97%) were born at term. At or near term, maternal hemoglobin was significantly higher (+5.56 g/L) for iron vs. placebo groups. Anemia risk was reduced (RR: 0.53; 95% CI: 0.43, 0.66), as were risks of ID (RR: 0.74; 95% CI: 0.69, 0.79 by SF; RR: 0.65; 95% CI: 0.59, 0.71 by BI) and IDA (RR: 0.49; 95% CI: 0.38, 0.62 by SF; RR: 0.51; 95% CI: 0.40, 0.65 by BI). Most women still had ID (66.8% by SF, 54.7% by BI). Adverse effects, all minor, were similar by group. There were no differences in cord blood iron measures; >45% of neonates in each group had ID. However, dose-response analyses showed higher cord SF with more maternal iron capsules reported being consumed (β per 10 capsules = 2.60, P < 0.05).
Conclusions: Prenatal iron supplementation reduced anemia, ID, and IDA in pregnant women in rural China, but most women and >45% of neonates had ID, regardless of supplementation. This trial was registered at clinicaltrials.gov as NCT02221752.
Keywords: iron deficiency; iron deficiency anemia; iron supplementation; neonates; pregnant women; randomized clinical trial.
© 2015 American Society for Nutrition.
Conflict of interest statement
Author disclosures: G Zhao, G Xu, M Zhou, Y Jiang, B Richards, KM Clark, N Kaciroti, MK Georgieff, Z Zhang, T Tardif, M Li, and B Lozoff, no conflicts of interest.
Figures

References
-
- Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Pena-Rosas JP, Bhutta ZA, Ezzati M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 2013;1:e16–25. - PMC - PubMed
-
- Dalenius K, Brindley P, Smith B, Reinold C, Grummer-Strawn L. Pregnancy Nutrition Surveillance 2010 Report. Atlanta (GA): US Department of Health and Human Services, CDC; 2012.
-
- Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr 2011;93:1312–20. - PubMed
-
- Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 2000;71(5, Suppl):1280S–4S. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

