Interaction of Vaccination and Reduction of Antibiotic Use Drives Unexpected Increase of Pneumococcal Meningitis
- PMID: 26063589
- PMCID: PMC4462765
- DOI: 10.1038/srep11293
Interaction of Vaccination and Reduction of Antibiotic Use Drives Unexpected Increase of Pneumococcal Meningitis
Abstract
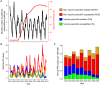
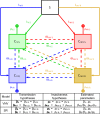
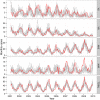
Antibiotic-use policies may affect pneumococcal conjugate-vaccine effectiveness. The reported increase of pneumococcal meningitis from 2001 to 2009 in France, where a national campaign to reduce antibiotic use was implemented in parallel to the introduction of the 7-valent conjugate vaccine, provides unique data to assess these effects. We constructed a mechanistic pneumococcal transmission model and used likelihood to assess the ability of competing hypotheses to explain that increase. We find that a model integrating a fitness cost of penicillin resistance successfully explains the overall and age-stratified pattern of serotype replacement. By simulating counterfactual scenarios of public health interventions in France, we propose that this fitness cost caused a gradual and pernicious interaction between the two interventions by increasing the spread of nonvaccine, penicillin-susceptible strains. More generally, our results indicate that reductions of antibiotic use may counteract the benefits of conjugate vaccines introduced into countries with low vaccine-serotype coverages and high-resistance frequencies. Our findings highlight the key role of antibiotic use in vaccine-induced serotype replacement and suggest the need for more integrated approaches to control pneumococcal infections.
Conflict of interest statement
Emmanuelle Varon received support for travel to meetings from Wyeth/Pfizer and Bayer, and was a speaker for Pfizer. Other authors involved in this study declare having no competing interests.
Figures

References
-
- Black S. et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19, 187–195 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

