Innovating to enhance clinical data management using non-commercial and open source solutions across a multi-center network supporting inpatient pediatric care and research in Kenya
- PMID: 26063746
- PMCID: PMC4681113
- DOI: 10.1093/jamia/ocv028
Innovating to enhance clinical data management using non-commercial and open source solutions across a multi-center network supporting inpatient pediatric care and research in Kenya
Abstract
Objective: To share approaches and innovations adopted to deliver a relatively inexpensive clinical data management (CDM) framework within a low-income setting that aims to deliver quality pediatric data useful for supporting research, strengthening the information culture and informing improvement efforts in local clinical practice.
Materials and methods: The authors implemented a CDM framework to support a Clinical Information Network (CIN) using Research Electronic Data Capture (REDCap), a noncommercial software solution designed for rapid development and deployment of electronic data capture tools. It was used for collection of standardized data from case records of multiple hospitals' pediatric wards. R, an open-source statistical language, was used for data quality enhancement, analysis, and report generation for the hospitals.
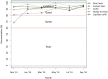
Results: In the first year of CIN, the authors have developed innovative solutions to support the implementation of a secure, rapid pediatric data collection system spanning 14 hospital sites with stringent data quality checks. Data have been collated on over 37 000 admission episodes, with considerable improvement in clinical documentation of admissions observed. Using meta-programming techniques in R, coupled with branching logic, randomization, data lookup, and Application Programming Interface (API) features offered by REDCap, CDM tasks were configured and automated to ensure quality data was delivered for clinical improvement and research use.
Conclusion: A low-cost clinically focused but geographically dispersed quality CDM (Clinical Data Management) in a long-term, multi-site, and real world context can be achieved and sustained and challenges can be overcome through thoughtful design and implementation of open-source tools for handling data and supporting research.
Keywords: clinical data management; clinical research; metaprogramming; open source; quality assurance.
© The Author 2015. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Figures

References
-
- Hannan EL . Randomized Clinical Trials and Observational StudiesGuidelines for Assessing Respective Strengths and Limitations .JACC: Cardiovasc Inte. 2008. ; 1 ( 3 ): 211 – 217 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

