Abnormal tuning of saccade-related cells in pontine reticular formation of strabismic monkeys
- PMID: 26063778
- PMCID: PMC4533063
- DOI: 10.1152/jn.00238.2015
Abnormal tuning of saccade-related cells in pontine reticular formation of strabismic monkeys
Abstract
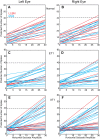
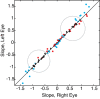
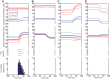
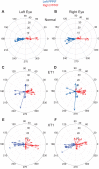
Strabismus is a common disorder, characterized by a chronic misalignment of the eyes and numerous visual and oculomotor abnormalities. For example, saccades are often highly disconjugate. For humans with pattern strabismus, the horizontal and vertical disconjugacies vary with eye position. In monkeys, manipulations that disturb binocular vision during the first several weeks of life result in a chronic strabismus with characteristics that closely match those in human patients. Early onset strabismus is associated with altered binocular sensitivity of neurons in visual cortex. Here we test the hypothesis that brain stem circuits specific to saccadic eye movements are abnormal. We targeted the pontine paramedian reticular formation, a structure that directly projects to the ipsilateral abducens nucleus. In normal animals, neurons in this structure are characterized by a high-frequency burst of spikes associated with ipsiversive saccades. We recorded single-unit activity from 84 neurons from four monkeys (two normal, one exotrope, and one esotrope), while they made saccades to a visual target on a tangent screen. All 24 neurons recorded from the normal animals had preferred directions within 30° of pure horizontal. For the strabismic animals, the distribution of preferred directions was normal on one side of the brain, but highly variable on the other. In fact, 12/60 neurons recorded from the strabismic animals preferred vertical saccades. Many also had unusually weak or strong bursts. These data suggest that the loss of corresponding binocular vision during infancy impairs the development of normal tuning characteristics for saccade-related neurons in brain stem.
Keywords: PPRF; esotropia; exotropia; monkey; strabismus.
Copyright © 2015 the American Physiological Society.
Figures




References
-
- Bucci MP, Kapoula Z, Yang Q, Roussat B, Bremond-Gignac D. Binocular coordination of saccades in children with strabismus before and after surgery. Invest Ophthalmol Vis Sci 43: 1040–1047, 2002. - PubMed
-
- Busettini C, Mays LE. Saccade-vergence interactions in macaques. II. Vergence enhancement as the product of a local feedback vergence motor error and a weighted saccadic burst. J Neurophysiol 94: 2312–2330, 2005. - PubMed
-
- Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med 19: 1141–1164, 2000. - PubMed
-
- Chu L, Kaneko CR. Do the saccadic system and the vestibulo-ocular reflex of monkeys share a common neural integrator? Neurosci Lett 187: 193–196, 1995. - PubMed
-
- Cohen B, Komatsuzaki A. Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp Neurol 36: 101–117, 1972. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

