Prohibitin: A Novel Molecular Player in KDEL Receptor Signalling
- PMID: 26064897
- PMCID: PMC4442004
- DOI: 10.1155/2015/319454
Prohibitin: A Novel Molecular Player in KDEL Receptor Signalling
Abstract
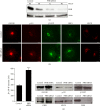
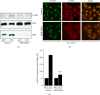
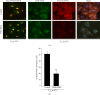
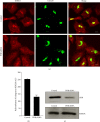
The KDEL receptor (KDELR) is a seven-transmembrane-domain protein involved in retrograde transport of protein chaperones from the Golgi complex to the endoplasmic reticulum. Our recent findings have shown that the Golgi-localised KDELR acts as a functional G-protein-coupled receptor by binding to and activating Gs and Gq. These G proteins induce activation of PKA and Src and regulate retrograde and anterograde Golgi trafficking. Here we used an integrated coimmunoprecipitation and mass spectrometry approach to identify prohibitin-1 (PHB) as a KDELR interactor. PHB is a multifunctional protein that is involved in signal transduction, cell-cycle control, and stabilisation of mitochondrial proteins. We provide evidence that depletion of PHB induces intense membrane-trafficking activity at the ER-Golgi interface, as revealed by formation of GM130-positive Golgi tubules, and recruitment of p115, β-COP, and GBF1 to the Golgi complex. There is also massive recruitment of SEC31 to endoplasmic-reticulum exit sites. Furthermore, absence of PHB decreases the levels of the Golgi-localised KDELR, thus preventing KDELR-dependent activation of Golgi-Src and inhibiting Golgi-to-plasma-membrane transport of VSVG. We propose a model whereby in analogy to previous findings (e.g., the RAS-RAF signalling pathway), PHB can act as a signalling scaffold protein to assist in KDELR-dependent Src activation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

