Micafungin triggers caspase-dependent apoptosis in Candida albicans and Candida parapsilosis biofilms, including caspofungin non-susceptible isolates
- PMID: 26065323
- PMCID: PMC4601157
- DOI: 10.1080/21505594.2015.1027479
Micafungin triggers caspase-dependent apoptosis in Candida albicans and Candida parapsilosis biofilms, including caspofungin non-susceptible isolates
Abstract
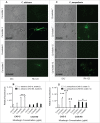
Candida biofilms play an important role in infections associated with medical devices and are resistant to antifungals. We hypothesized that the echinocandin micafungin (MICA) exerts an enhanced antifungal activity against caspofungin (CAS)-susceptible (CAS-S) and CAS-non-susceptible (CAS-NS) Candida albicans and Candida parapsilosis which is at least in part through apoptosis, even in the biofilm environment. Apoptosis was characterized by detecting reactive oxygen species (ROS) accumulation, depolarization of mitochondrial membrane potential (MMP), DNA fragmentation, lack of plasma membrane integrity, and metacaspase activation following exposure of Candida biofilm to MICA for 3h at 37°C in RPMI 1640 medium. The minimum inhibitory concentration was higher for CAS (2.0-16.0 μg/mL) than for MICA (1.0-8.0 μg/mL) for Candida biofilms. Elevated intracellular ROS levels and depolarization of MMP was evident in CAS-S C. albicans (3.0-4.2 fold) and C. parapsilosis (4.8-5.4 fold) biofilms compared with CAS-NS (1.2 fold) after exposure to MICA (0.25x-1xMIC). Elevated intracellular ROS levels and depolarization of MMP was evident in CAS-S C. albicans (3.0-4.2 fold) and C. parapsilosis (4.8-5.4 fold) biofilms compared with CAS-NS (1.2 fold) after exposure to MICA (0.25x-1xMIC). Finally higher ß-1, 3 glucan levels were seen in sessile cells compared to planktonic cells, especially in CAS-NS strains. MICA treatment might induce a metacaspase-dependent apoptotic process in biofilms of both CAS-S C. albicans and C. parapsilosis, and to some degree in CAS-NS strains.
Keywords: Apoptosis; biofilms; caspofungin; micafungin; reactive oxygen species.
Figures





References
-
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133-63; PMID:17223626; http://dx.doi.org/10.1128/CMR.00029-06 - DOI - PMC - PubMed
-
- Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, et al. . Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503-35; PMID:19191635; http://dx.doi.org/10.1086/596757 - DOI - PMC - PubMed
-
- Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev 2004; 17:255-67; PMID:15084500; http://dx.doi.org/10.1128/CMR.17.2.255-267.2004 - DOI - PMC - PubMed
-
- Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol 2003; 11:30-36; PMID:12526852; http://dx.doi.org/10.1016/S0966-842X(02)00002-1 - DOI - PubMed
-
- ten Cate JM, Klis FM, Pereira-Cenci T, Crielaard W, de Groot PW. Molecular and cellular mechanisms that lead to Candida biofilm formation. J Dent Res 2009; 88:105-15; PMID:19278980; http://dx.doi.org/10.1177/0022034508329273 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
