From cardiac tissue engineering to heart-on-a-chip: beating challenges
- PMID: 26065674
- PMCID: PMC4489846
- DOI: 10.1088/1748-6041/10/3/034006
From cardiac tissue engineering to heart-on-a-chip: beating challenges
Abstract
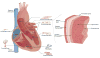
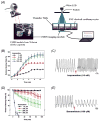
The heart is one of the most vital organs in the human body, which actively pumps the blood through the vascular network to supply nutrients to as well as to extract wastes from all other organs, maintaining the homeostasis of the biological system. Over the past few decades, tremendous efforts have been exerted in engineering functional cardiac tissues for heart regeneration via biomimetic approaches. More recently, progress has been made toward the transformation of knowledge obtained from cardiac tissue engineering to building physiologically relevant microfluidic human heart models (i.e. heart-on-chips) for applications in drug discovery. The advancement in stem cell technologies further provides the opportunity to create personalized in vitro models from cells derived from patients. Here, starting from heart biology, we review recent advances in engineering cardiac tissues and heart-on-a-chip platforms for their use in heart regeneration and cardiotoxic/cardiotherapeutic drug screening, and then briefly conclude with characterization techniques and personalization potential of the cardiac models.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
