Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics
- PMID: 26065697
- PMCID: PMC4699397
- DOI: 10.1038/ncomms8326
Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics
Abstract
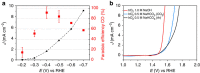
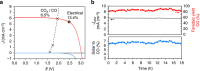
Artificial photosynthesis, mimicking nature in its efforts to store solar energy, has received considerable attention from the research community. Most of these attempts target the production of H2 as a fuel and our group recently demonstrated solar-to-hydrogen conversion at 12.3% efficiency. Here, in an effort to take this approach closer to real photosynthesis, which is based on the conversion of CO2, we demonstrate the efficient reduction of CO2 to carbon monoxide driven solely by simulated sunlight using water as the electron source. Employing series-connected perovskite photovoltaics and high-performance catalyst electrodes, we reach a solar-to-CO efficiency exceeding 6.5%, which represents a new benchmark in sunlight-driven CO2 conversion. Considering hydrogen as a secondary product, an efficiency exceeding 7% is observed. Furthermore, this study represents one of the first demonstrations of extended, stable operation of perovskite photovoltaics, whose large open-circuit voltage is shown to be particularly suited for this process.
Figures

References
-
- Marshall J. Solar energy: springtime for the artificial leaf. Nature 510, 22–24 (2014). - PubMed
-
- Khaselev O., Bansal A. & Turner J. A. High-efficiency integrated multijunction photovoltaic/electrolysis systems for hydrogen production. Int. J. Hydrog. Energy 26, 127–132 (2001).
-
- Luo J. et al. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 345, 1593–1596 (2014). - PubMed
-
- Mikkelsen M., Jorgensen M. & Krebs F. C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 3, 43–81 (2010).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

