γ-Secretase directly sheds the survival receptor BCMA from plasma cells
- PMID: 26065893
- PMCID: PMC4490565
- DOI: 10.1038/ncomms8333
γ-Secretase directly sheds the survival receptor BCMA from plasma cells
Abstract
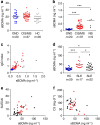
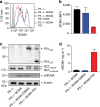
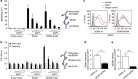
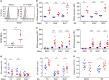
Survival of plasma cells is regulated by B-cell maturation antigen (BCMA), a membrane-bound receptor activated by its agonist ligands BAFF and APRIL. Here we report that γ-secretase directly cleaves BCMA, without prior truncation by another protease. This direct shedding is facilitated by the short length of BCMA's extracellular domain. In vitro, γ-secretase reduces BCMA-mediated NF-κB activation. In addition, γ-secretase releases soluble BCMA (sBCMA) that acts as a decoy neutralizing APRIL. In vivo, inhibition of γ-secretase enhances BCMA surface expression in plasma cells and increases their number in the bone marrow. Furthermore, in multiple sclerosis, sBCMA levels in spinal fluid are elevated and associated with intracerebral IgG production; in systemic lupus erythematosus, sBCMA levels in serum are elevated and correlate with disease activity. Together, shedding of BCMA by γ-secretase controls plasma cells in the bone marrow and yields a potential biomarker for B-cell involvement in human autoimmune diseases.
Figures




References
-
- Dörner T., Radbruch A. & Burmester G. R. B-cell-directed therapies for autoimmune disease. Nat. Rev. Rheumatol. 5, 433–441 (2009). - PubMed
-
- Krumbholz M., Derfuss T., Hohlfeld R. & Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat. Rev. Neurol. 8, 613–623 (2012). - PubMed
-
- Mackay F. & Schneider P. Cracking the BAFF code. Nat. Rev. Immunol. 9, 491–502 (2009). - PubMed
-
- Yang M. et al.. B cell maturation antigen, the receptor for a proliferation-inducing ligand and B cell-activating factor of the TNF family, induces antigen presentation in B cells. J. Immunol. 175, 2814–2824 (2005). - PubMed
-
- Darce J. R., Arendt B. K., Wu X. & Jelinek D. F. Regulated expression of BAFF-binding receptors during human B cell differentiation. J. Immunol. 179, 7276–7286 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

