Molecular snapshots of the Pex1/6 AAA+ complex in action
- PMID: 26066397
- PMCID: PMC4490564
- DOI: 10.1038/ncomms8331
Molecular snapshots of the Pex1/6 AAA+ complex in action
Abstract
The peroxisomal proteins Pex1 and Pex6 form a heterohexameric type II AAA+ ATPase complex, which fuels essential protein transport across peroxisomal membranes. Mutations in either ATPase in humans can lead to severe peroxisomal disorders and early death. We present an extensive structural and biochemical analysis of the yeast Pex1/6 complex. The heterohexamer forms a trimer of Pex1/6 dimers with a triangular geometry that is atypical for AAA+ complexes. While the C-terminal nucleotide-binding domains (D2) of Pex6 constitute the main ATPase activity of the complex, both D2 harbour essential substrate-binding motifs. ATP hydrolysis results in a pumping motion of the complex, suggesting that Pex1/6 function involves substrate translocation through its central channel. Mutation of the Walker B motif in one D2 domain leads to ATP hydrolysis in the neighbouring domain, giving structural insights into inter-domain communication of these unique heterohexameric AAA+ assemblies.
Figures



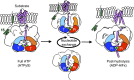
References
-
- van den Bosch H., Schutgens R. B., Wanders R. J. & Tager J. M. Biochemistry of peroxisomes. Annu. Rev. Biochem. 61, 157–197 (1992). - PubMed
-
- Voorn-Brouwer T., van der Leij I., Hemrika W., Distel B. & Tabak H. F. Sequence of the PAS8 gene, the product of which is essential for biogenesis of peroxisomes in Saccharomyces cerevisiae. Biochim. Biophys. Act 1216, 325–328 (1993). - PubMed
-
- Platta H. W., Grunau S., Rosenkranz K., Girzalsky W. & Erdmann R. Functional role of the AAA peroxins in dislocation of the cycling PTS1 receptor back to the cytosol. Nat. Cell Biol. 7, 817–822 (2005). - PubMed
-
- Reuber B. E. et al.. Mutations in PEX1 are the most common cause of peroxisome biogenesis disorders. Nat. Genet. 17, 445–448 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

