Analysis of Bottlenecks in Experimental Models of Infection
- PMID: 26066486
- PMCID: PMC4465827
- DOI: 10.1371/journal.ppat.1004823
Analysis of Bottlenecks in Experimental Models of Infection
Conflict of interest statement
The authors have declared that no competing interests exist.
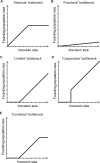
Figures

References
-
- Reece JB (2014) Campbell biology Tenth edition Boston: Pearson.
-
- Grenfell BT, Pybus OG, Gog JR, Wood JLN, Daly JM, et al. (2004) Unifying the epidemiological and evolutionary dynamics of pathogens. Science 303: 327–332. - PubMed
-
- Kouyos RD, Althaus CL, Bonhoeffer S (2006) Stochastic or deterministic: what is the effective population size of HIV-1? Trends Microbiol 14: 507–511. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

