Morphological plasticity of the coral skeleton under CO2-driven seawater acidification
- PMID: 26067341
- PMCID: PMC4490415
- DOI: 10.1038/ncomms8368
Morphological plasticity of the coral skeleton under CO2-driven seawater acidification
Abstract
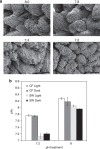
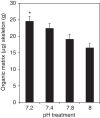
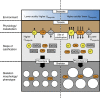
Ocean acidification causes corals to calcify at reduced rates, but current understanding of the underlying processes is limited. Here, we conduct a mechanistic study into how seawater acidification alters skeletal growth of the coral Stylophora pistillata. Reductions in colony calcification rates are manifested as increases in skeletal porosity at lower pH, while linear extension of skeletons remains unchanged. Inspection of the microstructure of skeletons and measurements of pH at the site of calcification indicate that dissolution is not responsible for changes in skeletal porosity. Instead, changes occur by enlargement of corallite-calyxes and thinning of associated skeletal elements, constituting a modification in skeleton architecture. We also detect increases in the organic matrix protein content of skeletons formed under lower pH. Overall, our study reveals that seawater acidification not only causes decreases in calcification, but can also cause morphological change of the coral skeleton to a more porous and potentially fragile phenotype.
Figures



References
-
- Knowlton N. et al.. in Life in the World's Oceans ed. Mcintyre A. Ch. 4, Wiley-Blackwell (2010).
-
- Caldeira K. & Wickett M. E. Oceanography: anthropogenic carbon and ocean pH. Nature 425, 365 (2003). - PubMed
-
- Orr J. C. et al.. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005). - PubMed
-
- Chan N. C. & Connolly S. R. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Chang. Biol. 19, 282–290 (2013). - PubMed
-
- Mumby P. J. & van Woesik R. Consequences of ecological, evolutionary and biogeochemical uncertainty for coral reef responses to climatic stress. Curr. Biol. 24, R413–R423 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

