A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain
- PMID: 26067585
- PMCID: PMC4770341
- DOI: 10.1097/j.pain.0000000000000263
A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain
Abstract
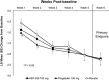
T-type Cav3.2 calcium channels represent a novel target for neuropathic pain modulation. Preclinical studies with ABT-639, a peripherally acting highly selective T-type Cav3.2 calcium channel blocker, showed dose-dependent reduction of pain in multiple pain models. ABT-639 also demonstrated an acceptable safety profile at single- and multiple-dose levels evaluated in a clinical phase 1 study in healthy volunteers. The primary objective of this phase 2, multicenter, randomized, double-blind, placebo-controlled, and active-controlled study was to compare the analgesic efficacy and safety of ABT-639 with placebo in the treatment of diabetic neuropathic pain. Pregabalin, an approved treatment for painful diabetic neuropathy, was included as a positive control. A total of 194 patients were randomized and treated for 6 weeks; 62 patients received ABT-639 (100 mg twice daily), 70 patients received pregabalin (150 mg twice daily), and 62 patients received placebo. When assessing the mean changes from baseline in patient-recorded pain scores at the end of week 6, there was no significant difference observed for ABT-639 compared with placebo (-2.28 vs -2.36; P = 0.582). Pregabalin treatment resulted in a transient improvement in pain compared with placebo, which did not persist throughout the study. There were no significant safety issues identified with ABT-639. A majority of adverse events were considered mild to moderate in intensity. In conclusion, treatment with the highly selective T-type Cav3.2 calcium channel blocker ABT-639 100 mg twice daily for 6 weeks showed no safety signals that would preclude further investigation but did not reduce neuropathic pain in patients with diabetes (ClinicalTrials.gov identifier: NCT01345045).
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
References
-
- Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 2003;302:1416–18. - PubMed
-
- Jarvis MF, Scott VE, McGaraughty S, Chu KL, Xu J, Niforatos W, Milicic I, Joshi S, Zhang Q, Xia Z. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. Biochem Pharmacol 2014;89:536–44. - PubMed
-
- Jarvis MF, Scott VE, McGaraughty S, Niforatos W, Milicic I, Joshi S, Zhang S, Xia Z. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. Presented at the Society for Neuroscience, November 13, 2013; Poster 829.01. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

