Simultaneous Pathoproteomic Evaluation of the Dystrophin-Glycoprotein Complex and Secondary Changes in the mdx-4cv Mouse Model of Duchenne Muscular Dystrophy
- PMID: 26067837
- PMCID: PMC4498307
- DOI: 10.3390/biology4020397
Simultaneous Pathoproteomic Evaluation of the Dystrophin-Glycoprotein Complex and Secondary Changes in the mdx-4cv Mouse Model of Duchenne Muscular Dystrophy
Abstract
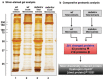
In skeletal muscle, the dystrophin-glycoprotein complex forms a membrane-associated assembly of relatively low abundance, making its detailed proteomic characterization in normal versus dystrophic tissues technically challenging. To overcome this analytical problem, we have enriched the muscle membrane fraction by a minimal differential centrifugation step followed by the comprehensive label-free mass spectrometric analysis of microsomal membrane preparations. This organelle proteomic approach successfully identified dystrophin and its binding partners in normal versus dystrophic hind limb muscles. The introduction of a simple pre-fractionation step enabled the simultaneous proteomic comparison of the reduction in the dystrophin-glycoprotein complex and secondary changes in the mdx-4cv mouse model of dystrophinopathy in a single analytical run. The proteomic screening of the microsomal fraction from dystrophic hind limb muscle identified the full-length dystrophin isoform Dp427 as the most drastically reduced protein in dystrophinopathy, demonstrating the remarkable analytical power of comparative muscle proteomics. Secondary pathoproteomic expression patterns were established for 281 proteins, including dystrophin-associated proteins and components involved in metabolism, signalling, contraction, ion-regulation, protein folding, the extracellular matrix and the cytoskeleton. Key findings were verified by immunoblotting. Increased levels of the sarcolemmal Na+/K+-ATPase in dystrophic leg muscles were also confirmed by immunofluorescence microscopy. Thus, the reduction of sample complexity in organelle-focused proteomics can be advantageous for the profiling of supramolecular protein complexes in highly intricate systems, such as skeletal muscle tissue.
Keywords: Na+/K+-ATPase; dystroglycan; dystrophin; dystrophinopathy; myozenin; organelle proteomics; periostin; sarcoglycan; syntrophin; tubulin.
Figures







References
-
- Højlund K., Yi Z., Hwang H., Bowen B., Lefort N., Flynn C.R., Langlais P., Weintraub S.T., Mandarino L.J. Characterization of the human skeletal muscle proteome by one-dimensional gel electrophoresis and HPLC-ESI-MS/MS. Mol. Cell. Proteomics. 2008;7:257–267. doi: 10.1074/mcp.M700304-MCP200. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

