Total and Proteinase K-Resistant α-Synuclein Levels in Erythrocytes, Determined by their Ability to Bind Phospholipids, Associate with Parkinson's Disease
- PMID: 26068055
- PMCID: PMC4464148
- DOI: 10.1038/srep11120
Total and Proteinase K-Resistant α-Synuclein Levels in Erythrocytes, Determined by their Ability to Bind Phospholipids, Associate with Parkinson's Disease
Abstract
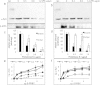
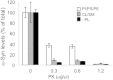
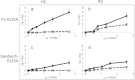
A marker for diagnosis of Parkinson's disease (PD), which reflects on the occurrence of peripheral pathogenic mechanisms, would potentially improve therapy. The significance of α-Synuclein (α-Syn) expression in red blood cells (RBC) is currently unclear. Here we investigated whether RBC's-expressed α-Syn may associate with PD. To this aim, we determined the levels of total and proteinase K-resistant α-Syn in samples of packed red blood cells (PRBCs). Twenty-one individuals with PD at various disease stages and 15 healthy controls, with similar demographic features, were recruited to this study. α-Syn levels were determined by their biochemical property to bind phospholipids, using a phospholipid-ELISA assay. A significantly lower ratio of total-to-proteinase K-resistant α-Syn levels was detected in PD patients than in the healthy control group. However, there was considerable overlap between the two groups. Suggesting a need for additional markers to be tested in combination with α-Syn levels. To the best of our knowledge, this is the first evidence for an association between RBCs-expressed α-Syn and pathogenic mechanisms involved in PD.
Conflict of interest statement
Dr. Ronit Sharon, PCT no. PCT/IL2014/050191: An ELISA method for a sensitive detection of alpha synuclein, consisting of its phospholipids binding properties.
Figures




References
-
- Goedert M., Spillantini M. G., Del Tredici K. & Braak H. 100 years of Lewy pathology. Nat Rev Neurol 9, 13–24 (2013). - PubMed
-
- Braak H., Ghebremedhin E., Rub U., Bratzke H. & Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318, 121–134 (2004). - PubMed
-
- Braak H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24, 197–211 (2003). - PubMed
-
- Olanow C. W., Wakeman D. R. & Kordower J. H. Peripheral alpha-synuclein and Parkinson’s disease. Mov Disord 29, 963–966 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

