A multi-center, open-label trial to compare the efficacy and pharmacokinetics of Artemether-Lumefantrine in children with severe acute malnutrition versus children without severe acute malnutrition: study protocol for the MAL-NUT study
- PMID: 26068100
- PMCID: PMC4464227
- DOI: 10.1186/s12879-015-0963-3
A multi-center, open-label trial to compare the efficacy and pharmacokinetics of Artemether-Lumefantrine in children with severe acute malnutrition versus children without severe acute malnutrition: study protocol for the MAL-NUT study
Abstract
Background: Malnutrition and malaria frequently coexist in sub-Saharan African countries. Studies on efficacy of antimalarial treatments usually follow the WHO standardized protocol in which severely malnourished children are systematically excluded. Few studies have assessed the efficacy of chloroquine, sulfadoxine-pyrimethamine and quinine in severe acute malnourished children. Overall, efficacy of these treatments appeared to be reduced, attributed to lower immunity and for some antimalarials altered pharmacokinetic profiles and lower drug concentrations. However, similar research on the efficacy and pharmacokinetic profiles of artemisinin-combination therapies (ACTs) and especially artemether-lumefantrine in malnourished children is currently lacking. The main objective of this study is to assess whether artemether-lumefantrine is less efficacious in children suffering from severe acute malnutrition (SAM) compared to non-SAM children, and if so, to what extent this can be attributed to a sub-optimal pharmacokinetic profile.
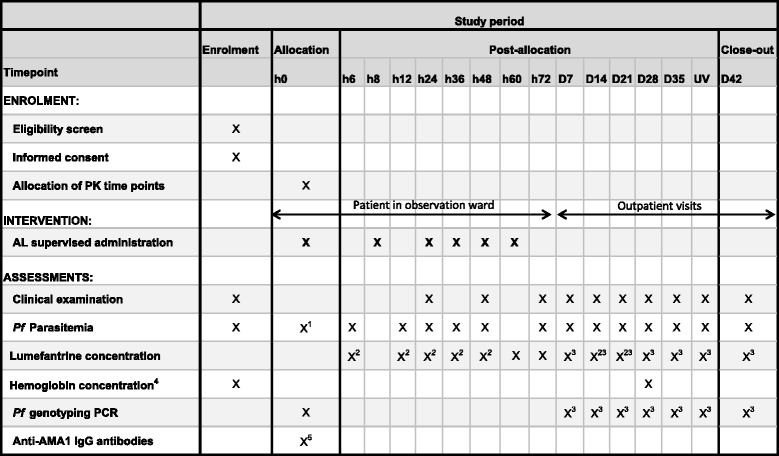
Methods/design: In two sites, Ouelessebougou, Mali and Maradi, Niger, children with uncomplicated microscopically-confirmed P. falciparum malaria aged between 6 and 59 months will be enrolled. Two non-SAM children will be enrolled after the enrolment of each SAM case. Children with severe manifestations of malaria or complications of acute malnutrition needing intensive treatment will be excluded. Treatment intakes will be supervised and children will be followed-up for 42 days, according to WHO guidance for surveillance of antimalarial drug efficacy. Polymerase Chain Reaction genotyping will be used to distinguish recrudescence from re-infection. SAM children will also benefit from the national nutritional rehabilitation program. Outcomes will be compared between the SAM and non-SAM populations. The primary outcome will be adequate clinical and parasitological response at day 28 after PCR correction, estimated by Kaplan-Meier analysis. To assess the pharmacokinetic profile of lumefantrine, a sparse sampling approach will be used with randomized allocation of sampling times (5 per child). A total of 180 SAM children and 360 non-SAM children will be recruited during the 2013 and 2014 malaria seasons.
Discussion: This study will provide important information that is currently lacking on the effect of SAM on therapeutic efficacy and pharmacokinetic profile of artemether-lumefantrine. If it shows lower therapeutic efficacy and decreased lumefantrine concentrations, it would inform dose optimization studies in SAM children.
Trial registration: ClinicalTrials.gov: NCT01958905.
Figures
References
-
- WHO . World Malaria Report. 2012.
-
- WHO/UNICEF. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children . WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children. 2009. - PubMed
-
- WHO Multicentre Growth Reference Study Group. WHO Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development. Geneva; 2006.
-
- Caulfield LE, Richard SA, Black RE. Undernutrition as an underlying cause of malaria morbidity and mortality in children less than five years old. Am J Trop Med Hyg. 2004;71(2 Suppl):55–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

