1α, 25-Dihydroxyvitamin D₃ and the vitamin D receptor regulates ΔNp63α levels and keratinocyte proliferation
- PMID: 26068789
- PMCID: PMC4669830
- DOI: 10.1038/cddis.2015.148
1α, 25-Dihydroxyvitamin D₃ and the vitamin D receptor regulates ΔNp63α levels and keratinocyte proliferation
Abstract
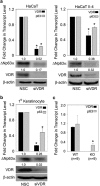
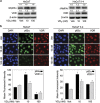
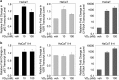
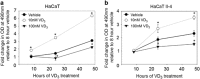
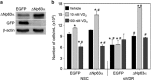
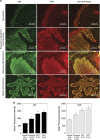
1α, 25-dihydroxyvitamin D3 (VD3), a secosteriod that has been explored as an anti-cancer agent, was also shown to promote cell survival. Its receptor, the Vitamin D Receptor (VDR), is a direct target of the proto-oncogene ΔNp63α, which is overexpressed in non-melanoma skin cancers. The interconnection between VDR/VD3 signaling and ΔNp63α, led us to examine whether VDR/VD3 signaling promotes keratinocyte proliferation by regulating ΔNp63α levels. Our data demonstrate that VDR regulates ΔNp63α expression at both the transcript and protein level. Interestingly, although low doses of VD3 led to an increase in ΔNp63α protein levels and keratinocyte proliferation, high doses of VD3 failed to increase ΔNp63α protein levels and resulted in reduced proliferation. Increased expression of ΔNp63α by low dose VD3 was shown to be dependent on VDR and critical for the proliferative effects of VD3. VD3-mediated increases in ΔNp63α protein levels occur via activation of both p38 MAPK and Akt kinases. Finally, analysis of samples from patients with squamous cell carcinoma (SCC), basal cell carcinoma and precursors to invasive SCC demonstrated a significant correlation between p63 and VDR levels when compared with healthy normal skin control samples. Delineation of the mechanisms by which VD3 exerts its effect on ΔNp63α and cell proliferation is critical for determining the future of VD3 in cancer therapies.
Figures


References
-
- Jin CH, Kerner SA, Hong MH, Pike JW. Transcriptional activation and dimerization functions in the human vitamin D receptor. Mol Endocrinol 1996; 10: 945–957. - PubMed
-
- Asagami C, Muto M, Hirota T, Shimizu T, Hamamoto Y. Anti-tumor effects of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) in seborrheic keratosis. J Investig Dermatol Symp Proc 1996; 1: 94–96. - PubMed
-
- Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007; 7: 684–700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

