Δ122p53, a mouse model of Δ133p53α, enhances the tumor-suppressor activities of an attenuated p53 mutant
- PMID: 26068791
- PMCID: PMC4669831
- DOI: 10.1038/cddis.2015.149
Δ122p53, a mouse model of Δ133p53α, enhances the tumor-suppressor activities of an attenuated p53 mutant
Abstract
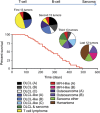
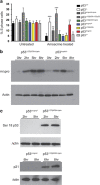
Growing evidence suggests the Δ133p53α isoform may function as an oncogene. It is overexpressed in many tumors, stimulates pathways involved in tumor progression, and inhibits some activities of wild-type p53, including transactivation and apoptosis. We hypothesized that Δ133p53α would have an even more profound effect on p53 variants with weaker tumor-suppressor capability. We tested this using a mouse model heterozygous for a Δ133p53α-like isoform (Δ122p53) and a p53 mutant with weak tumor-suppressor function (mΔpro). The Δ122p53/mΔpro mice showed a unique survival curve with a wide range of survival times (92-495 days) which was much greater than mΔpro/- mice (range 120-250 days) and mice heterozygous for the Δ122p53 and p53 null alleles (Δ122p53/-, range 78-150 days), suggesting Δ122p53 increased the tumor-suppressor activity of mΔpro. Moreover, some of the mice that survived longest only developed benign tumors. In vitro analyses to investigate why some Δ122p53/mΔpro mice were protected from aggressive tumors revealed that Δ122p53 stabilized mΔpro and prolonged the response to DNA damage. Similar effects of Δ122p53 and Δ133p53α were observed on wild-type of full-length p53, but these did not result in improved biological responses. The data suggest that Δ122p53 (and Δ133p53α) could offer some protection against tumors by enhancing the p53 response to stress.
Figures




References
-
- Braithwaite AW, Del Sal G, Lu X. Some p53-binding proteins that can function as arbiters of life and death. Cell Death Differ 2006; 13: 984–993. - PubMed
-
- Braithwaite AW, Prives CL. p53: more research and more questions. Cell Death Differ 2006; 13: 877–880. - PubMed
-
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10: 431–442. - PubMed
-
- Speidel D. The role of DNA damage responses in p53 biology. Arch Toxicol 2015; 89: 501–517. - PubMed
-
- Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 2006; 126: 107–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

