Effect of Traumatic Brain Injury, Erythropoietin, and Anakinra on Hepatic Metabolizing Enzymes and Transporters in an Experimental Rat Model
- PMID: 26068867
- PMCID: PMC4540723
- DOI: 10.1208/s12248-015-9792-y
Effect of Traumatic Brain Injury, Erythropoietin, and Anakinra on Hepatic Metabolizing Enzymes and Transporters in an Experimental Rat Model
Abstract
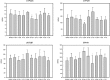
In contrast to considerable data demonstrating a decrease in cytochrome P450 (CYP) activity in inflammation and infection, clinically, traumatic brain injury (TBI) results in an increase in CYP and UDP glucuronosyltransferase (UGT) activity. The objective of this study was to determine the effects of TBI alone and with treatment with erythropoietin (EPO) or anakinra on the gene expression of hepatic inflammatory proteins, drug-metabolizing enzymes, and transporters in a cortical contusion impact (CCI) injury model. Microarray-based transcriptional profiling was used to determine the effect on gene expression at 24 h, 72 h, and 7 days post-CCI. Plasma cytokine and liver protein concentrations of CYP2D4, CYP3A1, EPHX1, and UGT2B7 were determined. There was no effect of TBI, TBI + EPO, or TBI + anakinra on gene expression of the inflammatory factors shown to be associated with decreased expression of hepatic metabolic enzymes in models of infection and inflammation. IL-6 plasma concentrations were increased in TBI animals and decreased with EPO and anakinra treatment. There was no significant effect of TBI and/or anakinra on gene expression of enzymes or transporters known to be involved in drug disposition. TBI + EPO treatment decreased the gene expression of Cyp2d4 at 72 h with a corresponding decrease in CYP2D4 protein at 72 h and 7 days. CYP3A1 protein was decreased at 24 h. In conclusion, EPO treatment may result in a significant decrease in the metabolism of Cyp-metabolized drugs. In contrast to clinical TBI, there was not a significant effect of experimental TBI on CYP or UGT metabolic enzymes.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

