Eilat virus induces both homologous and heterologous interference
- PMID: 26068885
- PMCID: PMC4567418
- DOI: 10.1016/j.virol.2015.05.009
Eilat virus induces both homologous and heterologous interference
Abstract
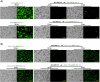
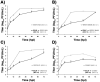
Most alphaviruses are mosquito-borne and exhibit a broad host range, infecting many different vertebrates including birds, rodents, equids, and humans. Occasionally, alphaviruses can spill over into the human population and cause disease characterized by debilitating arthralgia or fatal encephalitis. Recently, a unique alphavirus, Eilat virus (EILV), was described that readily infects mosquito but not vertebrate cell lines. Here, we investigated the ability of EILV to induce superinfection exclusion. Prior infection of C7/10 (Aedes albopictus) cells with EILV induced homologous and heterologous interference, reducing the virus titers of heterologous superinfecting viruses (SINV, VEEV, EEEV, WEEV, and CHIKV) by ~10-10,000 fold and delaying replication kinetics by 12-48h. Similar to in vitro infection, prior in vivo EILV infection of Aedes aegypti mosquitoes delayed dissemination of chikungunya virus for 3 days. This is the first evidence of heterologous interference induced by a mosquito-specific alphavirus in vitro and in vivo.
Keywords: Alphavirus; Eilat virus; Superinfection.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



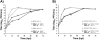

References
-
- Altman RM. The behavior of Murray Valley encephalitis virus in Culex tritaeniorhynchus Giles and Culex pipiens quinquefasciatus Say. Am J Trop Med Hyg. 1963;12:425–434. - PubMed
-
- Beaty BJ, Bishop DHL, Gay M, Fuller F. Interference between Bunyaviruses in Aedes triseriatus mosquitoes. Virology. 1983;127:83–90. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

