Zoledronic acid as compared with observation in multiple myeloma patients at biochemical relapse: results of the randomized AZABACHE Spanish trial
- PMID: 26069291
- PMCID: PMC4800701
- DOI: 10.3324/haematol.2015.128439
Zoledronic acid as compared with observation in multiple myeloma patients at biochemical relapse: results of the randomized AZABACHE Spanish trial
Abstract
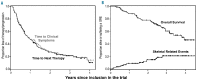
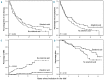
This study analyzed the anti-myeloma effect of zoledronic acid monotherapy by investigating patients at the time of asymptomatic biochemical relapse. One hundred patients were randomized to receive either zoledronic acid (4 mg iv/4 weeks, 12 doses) (n=51) or not (n=49). Experimental and control groups were well balanced for disease and prognostic features. Zoledronic acid did not show an antitumor effect according to changes in M-component. However, there were fewer symptomatic progressions in the experimental group than in the control group (34 versus 41, respectively; P=0.05) resulting in a median time to symptoms of 16 versus 10 months (P=0.161). The median time to next therapy was also slightly longer for the treated group than the untreated, control group (13.4 versus 10.1 months), although the difference was not statistically significant (P=0.360). The pattern of relapses was different for treated versus control patients: progressive bone disease (8 versus 20), anemia (24 versus 18), renal dysfunction (1 versus 2), and plasmacytomas (1 versus 1, respectively). This concurred with fewer skeletal-related events in the treated group than in the control group (2 versus 14), with a projected 4-year event proportion of 6% versus 40% (P<0.001). In summary, zoledronic acid monotherapy does not show an antitumor effect on biochemical relapses in multiple myeloma, but does reduce the risk of progression with symptomatic bone disease and skeletal complications. This trial was registered in the ClinicalTrials.gov database with code NCT01087008.
Copyright© Ferrata Storti Foundation.
Figures

References
-
- Mateos M-V, San Miguel JF. How should we treat newly diagnosed multiple myeloma patients? Hematology Am Soc Hematol Educ Program. 2013;2013:488–495. - PubMed
-
- Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9): 1467–1473. - PubMed
-
- Croucher PI, De Hendrik R, Perry MJ, et al. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 2003;18(3):482–492. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

