International Cartilage Repair Society (ICRS) Recommended Guidelines for Histological Endpoints for Cartilage Repair Studies in Animal Models and Clinical Trials
- PMID: 26069577
- PMCID: PMC4300784
- DOI: 10.1177/1947603510397535
International Cartilage Repair Society (ICRS) Recommended Guidelines for Histological Endpoints for Cartilage Repair Studies in Animal Models and Clinical Trials
Abstract
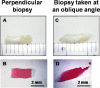
Cartilage repair strategies aim to resurface a lesion with osteochondral tissue resembling native cartilage, but a variety of repair tissues are usually observed. Histology is an important structural outcome that could serve as an interim measure of efficacy in randomized controlled clinical studies. The purpose of this article is to propose guidelines for standardized histoprocessing and unbiased evaluation of animal tissues and human biopsies. Methods were compiled from a literature review, and illustrative data were added. In animal models, treatments are usually administered to acute defects created in healthy tissues, and the entire joint can be analyzed at multiple postoperative time points. In human clinical therapy, treatments are applied to developed lesions, and biopsies are obtained, usually from a subset of patients, at a specific time point. In striving to standardize evaluation of structural endpoints in cartilage repair studies, 5 variables should be controlled: 1) location of biopsy/sample section, 2) timing of biopsy/sample recovery, 3) histoprocessing, 4) staining, and 5) blinded evaluation with a proper control group. Histological scores, quantitative histomorphometry of repair tissue thickness, percentage of tissue staining for collagens and glycosaminoglycan, polarized light microscopy for collagen fibril organization, and subchondral bone integration/structure are all relevant outcome measures that can be collected and used to assess the efficacy of novel therapeutics. Standardized histology methods could improve statistical analyses, help interpret and validate noninvasive imaging outcomes, and permit cross-comparison between studies. Currently, there are no suitable substitutes for histology in evaluating repair tissue quality and cartilaginous character.
Keywords: animal models; articular cartilage; biopsy; cartilage repair; collagen type I; collagen type II; fibrocartilage; glycosaminoglycan; histology; polarized light microscopy; subchondral bone; tidemark.
Conflict of interest statement
Figures




References
-
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-60. - PubMed
-
- Mithoefer K, McAdams TR, Scopp JM, Mandelbaum BR. Emerging options for treatment of articular cartilage injury in the athlete. Clin Sports Med. 2009;28(1):25-40. - PubMed
-
- Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91A(7):1778-90. - PubMed
-
- Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92A(4):994-1009. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

