Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis and survival
- PMID: 26069821
- PMCID: PMC4455827
- DOI: 10.1093/ndtplus/sfr167
Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis and survival
Abstract
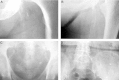
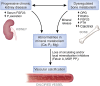
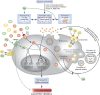
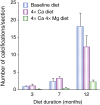
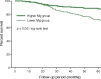
Patients with chronic kidney disease (CKD) have a high prevalence of vascular calcification, and cardiovascular disease is the leading cause of death in this population. However, the molecular mechanisms of vascular calcification, which are multifactorial, cell-mediated and dynamic, are not yet fully understood. We need to address ways to improve outcomes in CKD patients, both in terms of vascular calcification and cardiovascular morbidity and mortality-and to these ends, we investigate the role of magnesium. Magnesium's role in the pathogenesis of vascular calcification has not been extensively studied. Nonetheless, several in vitro and animal studies point towards a protective role of magnesium through multiple molecular mechanisms. Magnesium is a natural calcium antagonist and both human and animal studies have shown that low circulating magnesium levels are associated with vascular calcification. Clinical evidence from observational studies of dialysis patients has shown that low-magnesium levels occur concurrently with mitral annular calcification, peripheral arterial calcification and increased carotid intima-media thickness. Few interventional studies have been performed. Two interventional studies suggest that there may be benefits such as retardation of arterial calcification and/or reductions in carotid intima-media thickness in response to magnesium supplementation in CKD patients, though both studies have limitations. Finally, observational studies have shown that low serum magnesium may be an independent risk factor for premature death in CKD patients, and patients with mildly elevated serum magnesium levels could have a survival advantage over those with lower magnesium levels.
Keywords: atherosclerosis; chronic kidney disease; magnesium; survival; vascular calcification.
Figures
Similar articles
-
Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification.Am J Nephrol. 2012;35(1):31-9. doi: 10.1159/000334742. Epub 2011 Dec 15. Am J Nephrol. 2012. PMID: 22179063
-
Low Magnesium Levels and FGF-23 Dysregulation Predict Mitral Valve Calcification as well as Intima Media Thickness in Predialysis Diabetic Patients.Int J Endocrinol. 2015;2015:308190. doi: 10.1155/2015/308190. Epub 2015 May 18. Int J Endocrinol. 2015. PMID: 26089881 Free PMC article.
-
Magnesium and cardiovascular complications of chronic kidney disease.Nat Rev Nephrol. 2015 Jul;11(7):432-42. doi: 10.1038/nrneph.2015.74. Epub 2015 May 12. Nat Rev Nephrol. 2015. PMID: 25963594 Review.
-
Valvular calcification and its relationship to atherosclerosis in chronic kidney disease.J Heart Valve Dis. 2009 Jul;18(4):429-38. J Heart Valve Dis. 2009. PMID: 19852148
-
Magnesium and Vascular Calcification in Chronic Kidney Disease: Current Insights.Int J Mol Sci. 2024 Jan 18;25(2):1155. doi: 10.3390/ijms25021155. Int J Mol Sci. 2024. PMID: 38256228 Free PMC article. Review.
Cited by
-
Inquiry of the Metabolic Traits in Relationship with Daily Magnesium Intake: Focus on Type 2 Diabetic Population.Clin Pract. 2024 Jul 8;14(4):1319-1347. doi: 10.3390/clinpract14040107. Clin Pract. 2024. PMID: 39051301 Free PMC article. Review.
-
Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells.Nephrol Dial Transplant. 2013 Apr;28(4):869-78. doi: 10.1093/ndt/gfs520. Epub 2012 Dec 9. Nephrol Dial Transplant. 2013. PMID: 23229924 Free PMC article.
-
Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium.PLoS One. 2015 Jan 21;10(1):e0115342. doi: 10.1371/journal.pone.0115342. eCollection 2015. PLoS One. 2015. PMID: 25607936 Free PMC article. Review.
-
Use of magnesium as a drug in chronic kidney disease.Clin Kidney J. 2012 Feb;5(Suppl 1):i62-i70. doi: 10.1093/ndtplus/sfr168. Clin Kidney J. 2012. PMID: 26069822 Free PMC article.
-
Cardiovascular Calcification in Chronic Kidney Disease-Therapeutic Opportunities.Toxins (Basel). 2020 Mar 14;12(3):181. doi: 10.3390/toxins12030181. Toxins (Basel). 2020. PMID: 32183352 Free PMC article. Review.
References
-
- Foley RN, Parfrey PS. Cardiovascular disease and mortality in ESRD. J Nephrol. 1998;11:239–245. - PubMed
-
- Braun J, Oldendorf M, Moshage W, et al. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis. 1996;27:394–401. - PubMed
-
- Blacher J, Demuth K, Guerin AP, et al. Influence of biochemical alterations on arterial stiffness in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 1998;18:535–541. - PubMed
-
- Speer MY, Giachelli CM. Regulation of cardiovascular calcification. Cardiovasc Pathol. 2004;13:63–70. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

