Monitoring serum HER2 levels in breast cancer patients
- PMID: 26069876
- PMCID: PMC4456592
- DOI: 10.1186/s40064-015-1015-6
Monitoring serum HER2 levels in breast cancer patients
Abstract
Background: We have developed a new approach to reduce the serum interference for ELISA. The purpose of this study is to investigate if we can use the optimized ELISA (MBB-ELISA) to detect serum soluble HER2/neu (sHER2) in early stage primary breast cancer and monitor its change during treatments.
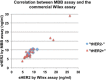
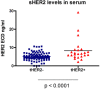
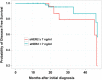
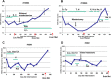
Findings: We collected sera preoperatively from 118 primary breast cancer patients. Serum samples were also collected sequentially from a subset of patients during and after adjuvant treatment. sHER2 in these samples was measured by the MBB-ELISA. Only 16.7 % of tissue HER2 (tHER2) positive patients had significantly elevated sHER2 levels in serum. Interestingly, sera of some patients with tHER2 negative tumors, including those that were 2+ by IHC but negative by FISH, demonstrated slightly elevated sHER2 levels. Multivariate analysis demonstrated that patients with elevated sHER2 (> = 7 ng/ml) had significantly worse disease free survival. During treatments, sHER2 levels consistently fell in response to adjuvant therapies. Nevertheless, in all 4 patients who developed metastases, a steady rise in sHER2 levels was noted before metastatic disease became clinically evident.
Conclusions: For early stage breast cancers, sHER2 is a poor biomarker to predict tHER2 status, but may have value to supplement tissue tests to identify patients with HER2 tumors. Our results also suggest that sHER2 is worth further study as a biomarker to monitor breast cancer patients during treatments.
Keywords: Biomarker; Breast cancer; HER2/neu; MBB buffer; SHER2.
Figures
Similar articles
-
Pilot Study on the Utility of Circulating HER2/Neu Levels in the Serum of Breast Cancer Patients.Anticancer Res. 2019 Oct;39(10):5345-5352. doi: 10.21873/anticanres.13727. Anticancer Res. 2019. PMID: 31570428
-
Serum Human Epidermal Growth Factor 2 Extracellular Domain as a Predictive Biomarker for Lapatinib Treatment Efficacy in Patients With Advanced Breast Cancer.J Clin Oncol. 2016 Mar 20;34(9):936-44. doi: 10.1200/JCO.2015.62.4767. Epub 2016 Jan 25. J Clin Oncol. 2016. PMID: 26811533
-
Assessing neoadjuvant treatment response through serum human epidermal growth factor receptor 2 (HER2) dynamics.Gland Surg. 2025 Feb 28;14(2):207-218. doi: 10.21037/gs-24-432. Epub 2025 Feb 25. Gland Surg. 2025. PMID: 40115849 Free PMC article.
-
The extracellular domain of Her2 in serum as a biomarker of breast cancer.Lab Invest. 2018 Jun;98(6):696-707. doi: 10.1038/s41374-018-0033-8. Epub 2018 Feb 28. Lab Invest. 2018. PMID: 29491426 Review.
-
HER2 shedding and serum HER2 extracellular domain: biology and clinical utility in breast cancer.Cancer Treat Rev. 2012 Apr;38(2):133-42. doi: 10.1016/j.ctrv.2011.03.008. Epub 2011 May 5. Cancer Treat Rev. 2012. PMID: 21549508 Review.
Cited by
-
Evaluation of HER2/neu oncoprotein in serum & tissue samples of women with breast cancer.Indian J Med Res. 2016 May;143(Supplement):S52-S58. doi: 10.4103/0971-5916.191769. Indian J Med Res. 2016. PMID: 27748278 Free PMC article.
-
Exosomes: A Promising Avenue for the Diagnosis of Breast Cancer.Technol Cancer Res Treat. 2019 Jan 1;18:1533033818821421. doi: 10.1177/1533033818821421. Technol Cancer Res Treat. 2019. PMID: 30760122 Free PMC article. Review.
-
Bloch Surface Waves Biosensors for High Sensitivity Detection of Soluble ERBB2 in a Complex Biological Environment.Biosensors (Basel). 2017 Aug 17;7(3):33. doi: 10.3390/bios7030033. Biosensors (Basel). 2017. PMID: 28817097 Free PMC article.
-
An updated evaluation of serum sHER2, CA15.3, and CEA levels as biomarkers for the response of patients with metastatic breast cancer to trastuzumab-based therapies.PLoS One. 2020 Jan 7;15(1):e0227356. doi: 10.1371/journal.pone.0227356. eCollection 2020. PLoS One. 2020. PMID: 31910438 Free PMC article.
-
Circulating proteins as predictive and prognostic biomarkers in breast cancer.Clin Proteomics. 2022 Jul 11;19(1):25. doi: 10.1186/s12014-022-09362-0. Clin Proteomics. 2022. PMID: 35818030 Free PMC article. Review.
References
-
- Christianson TA, Doherty JK, Lin YJ, Ramsey EE, Holmes R, Keenan EJ, Clinton GM. NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998;58(22):5123–5129. - PubMed
-
- Colomer R, Montero S, Lluch A, Ojeda B, Barnadas A, Casado A, Massuti B, Cortes-Funes H, Lloveras B. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 2000;6(6):2356–2362. - PubMed
-
- Esteva FJ, Cheli CD, Fritsche H, Fornier M, Slamon D, Thiel RP, Luftner D, Ghani F. Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res. 2005;7(4):R436–R443. doi: 10.1186/bcr1020. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

