HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins
- PMID: 26070106
- PMCID: PMC4629848
- DOI: 10.1021/acschembio.5b00442
HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins
Abstract
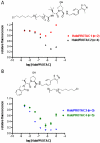
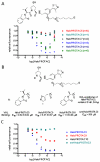
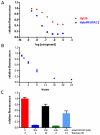
Small molecule-induced protein degradation is an attractive strategy for the development of chemical probes. One method for inducing targeted protein degradation involves the use of PROTACs, heterobifunctional molecules that can recruit specific E3 ligases to a desired protein of interest. PROTACs have been successfully used to degrade numerous proteins in cells, but the peptidic E3 ligase ligands used in previous PROTACs have hindered their development into more mature chemical probes or therapeutics. We report the design of a novel class of PROTACs that incorporate small molecule VHL ligands to successfully degrade HaloTag7 fusion proteins. These HaloPROTACs will inspire the development of future PROTACs with more drug-like properties. Additionally, these HaloPROTACs are useful chemical genetic tools, due to their ability to chemically knock down widely used HaloTag7 fusion proteins in a general fashion.
Figures




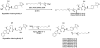
Comment in
-
Protein-slaying drugs could be the next blockbuster therapies.Nature. 2019 Mar;567(7748):298-300. doi: 10.1038/d41586-019-00879-3. Nature. 2019. PMID: 30894734 No abstract available.
References
-
- Banaszynski LA, Wandless TJ. Conditional control of protein function. Chem. Biol. 2006;13:11–21. - PubMed
-
- Schneekloth JS, Jr, Fonseca FN, Koldobskiy M, Mandal AK, Deshaies RJ, Sakamoto K, Crews CM. Chemical genetic control of protein levels: selective in vivo targeted degradation. J. Am. Chem. Soc. 2004;126:3748–3754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

