Point-of-care multiplex PCR promises short turnaround times for microbial testing in hospital-acquired pneumonia--an observational pilot study in critical ill patients
- PMID: 26071191
- PMCID: PMC4469099
- DOI: 10.1186/s12941-015-0091-3
Point-of-care multiplex PCR promises short turnaround times for microbial testing in hospital-acquired pneumonia--an observational pilot study in critical ill patients
Abstract
Background: The early beginning of an adequate antibiotic therapy is crucial in hospital-acquired pneumonia (HAP), but depends on the results of conventional microbiological diagnostics (cMD). It was the aim of this study to evaluate the performance and turnaround times of a new point-of-care multiplex polymerase chain reaction (mPCR) system for rapid identification of pathogens and antibiotic resistance markers. We assessed the applicability of the system under real-life conditions in critical ill patients with HAP.
Methods: We enrolled forty critical ill patients with clinical signs for HAP into an observational study. Two samples of respiratory secretions were collected during one course of aspiration and cMD and mPCR testing (Unyvero, Curetis AG, Holzgerlingen, Germany) were performed immediately. The mPCR device was operated as a point-of-care system at the intensive care unit. We compared turnaround times, results of pathogen identification and results of antibiotic resistance testing of both methods.
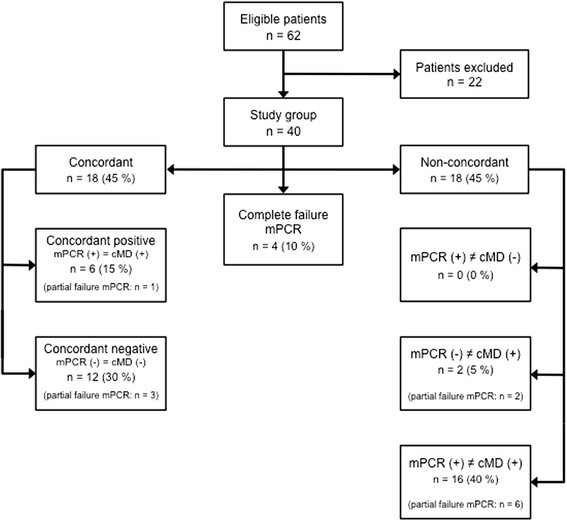
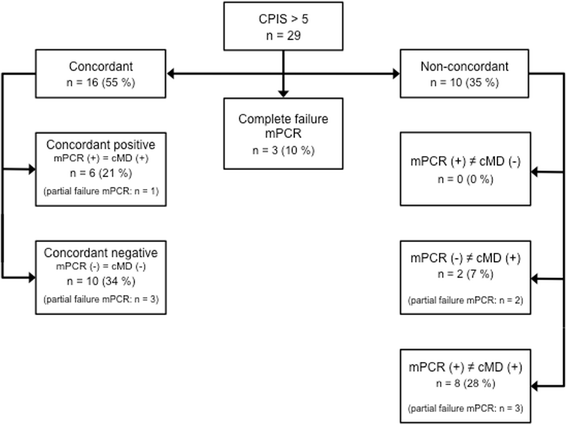
Results: Mean turnaround times (min-max) were 6.5 h (4.7-18.3 h) for multiplex PCR and 71 h (37.2-217.8 h) for conventional microbiology (final cMD results, incomplete results neglected). 60% (n = 24) of the mPCR tests were completely valid. Complete test failure occurred in 10% (n = 4) and partial test failure occurred in 30% (n = 12). We found concordant results in 45% (n = 18) and non-concordant results in 45% (n = 18) of all patients. 55% (n = 16) of the results were concordant in patients with a clinical pulmonary infection score (CPIS) > 5 (n = 29). Concordant results included three cases of multidrug resistant bacteria. MPCR frequently detected antibiotic resistance markers that were not found by cMD.
Conclusions: Unyvero allowed point-of-care microbial testing with short turnaround times. The performance of the system was poor. However, an improved system with a more reliable performance and an extended microbial panel could be a useful addition to cMD in intensive care medicine.
Trial registration: ClinicalTrials.gov NCT01858974 (registered 16 May 2013).
Figures
References
-
- van Veen SQ, Claas EC, Kuijper EJ. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J Clin Microbiol. 2010;48(3):900–7. doi: 10.1128/JCM.02071-09. - DOI - PMC - PubMed
-
- Kunze N, Gopel J, Kuhns M, Junger M, Quintel M, Perl T. Detection and validation of volatile metabolic patterns over different strains of two human pathogenic bacteria during their growth in a complex medium using multi-capillary column-ion mobility spectrometry (MCC-IMS) Appl Microbiol Biotechnol. 2013;97(8):3665–76. doi: 10.1007/s00253-013-4762-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials