Phase 2 Trial of an Alpha-7 Nicotinic Receptor Agonist (TC-5619) in Negative and Cognitive Symptoms of Schizophrenia
- PMID: 26071208
- PMCID: PMC4753586
- DOI: 10.1093/schbul/sbv072
Phase 2 Trial of an Alpha-7 Nicotinic Receptor Agonist (TC-5619) in Negative and Cognitive Symptoms of Schizophrenia
Abstract
Objectives: This trial was conducted to test the effects of an alpha7 nicotinic receptor full agonist, TC-5619, on negative and cognitive symptoms in subjects with schizophrenia.
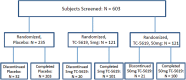
Methods: In 64 sites in the United States, Russia, Ukraine, Hungary, Romania, and Serbia, 477 outpatients (18-65 years; male 62%; 55% tobacco users) with schizophrenia, treated with a new-generation antipsychotic, were randomized to 24 weeks of placebo (n = 235), TC-5619, 5mg (n = 121), or TC-5619, 50 mg (n = 121), administered orally once daily. The primary efficacy measure was the Scale for the Assessment of Negative Symptoms (SANS) composite score. Key secondary measures were the Cogstate Schizophrenia Battery (CSB) composite score and the University of California San Diego Performance-Based Skills Assessment-Brief Version (UPSA-B) total score. Secondary measures included: Positive and Negative Syndrome Scale in Schizophrenia (PANSS) total and subscale scores, SANS domain scores, CSB item scores, Clinical Global Impression-Global Improvement (CGI-I) score, CGI-Severity (CGI-S) score, and Subject Global Impression-Cognition (SGI-Cog) total score.
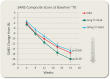
Results: SANS score showed no statistical benefit for TC-5619 vs placebo at week 24 (5 mg, 2-tailed P = .159; 50 mg, P = .689). Likewise, no scores of CSB, UPSA-B, PANSS, CGI-I, CGI-S, or SGI-Cog favored TC-5619 (P > .05). Sporadic statistical benefit favoring TC-5619 in some of these outcome measures were observed in tobacco users, but these benefits did not show concordance by dose, country, gender, or other relevant measures. TC-5619 was generally well tolerated.
Conclusion: These results do not support a benefit of TC-5619 for negative or cognitive symptoms in schizophrenia.
Keywords: cognition; negative symptoms; schizophrenia.
© The Author 2015. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, Virginia: APA; 2013.
-
- Marder SR, Fenton W. Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9. - PubMed
-
- Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res. 2004;72:21–28. - PubMed
-
- Stahl SM, Buckley PF. Negative symptoms of schizophrenia: a problem that will not go away. Acta Psychiatr Scand. 2007;115:4–11. - PubMed

