Alternative routes to the cell surface underpin insulin-regulated membrane trafficking of GLUT4
- PMID: 26071524
- PMCID: PMC4510850
- DOI: 10.1242/jcs.166561
Alternative routes to the cell surface underpin insulin-regulated membrane trafficking of GLUT4
Abstract
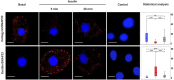
Insulin-stimulated delivery of glucose transporters (GLUT4, also known as SLC2A4) from specialized intracellular GLUT4 storage vesicles (GSVs) to the surface of fat and muscle cells is central to whole-body glucose regulation. This translocation and subsequent internalization of GLUT4 back into intracellular stores transits through numerous small membrane-bound compartments (internal GLUT4-containing vesicles; IGVs) including GSVs, but the function of these different compartments is not clear. Cellugyrin (also known as synaptogyrin-2) and sortilin define distinct populations of IGV; sortilin-positive IGVs represent GSVs, but the function of cellugyrin-containing IGVs is unknown. Here, we demonstrate a role for cellugyrin in intracellular sequestration of GLUT4 in HeLa cells and have used a proximity ligation assay to follow changes in pairwise associations between cellugyrin, sortilin, GLUT4 and membrane trafficking machinery following insulin-stimulation of 3T3-L1 adipoctyes. Our data suggest that insulin stimulates traffic from cellugyrin-containing to sortilin-containing membranes, and that cellugyrin-containing IGVs provide an insulin-sensitive reservoir to replenish GSVs following insulin-stimulated exocytosis of GLUT4. Furthermore, our data support the existence of a pathway from cellugyrin-containing membranes to the surface of 3T3-L1 adipocytes that bypasses GSVs under basal conditions, and that insulin diverts traffic away from this into GSVs.
Keywords: Endosome; Gyrin; Membrane traffic.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

