Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting
- PMID: 26072017
- PMCID: PMC5845829
- DOI: 10.1016/j.vaccine.2015.05.088
Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting
Abstract
Background: Vaccination that prevents tuberculosis (TB) disease, particularly in adolescents, would have the greatest impact on the global TB epidemic. Safety, reactogenicity and immunogenicity of the vaccine candidate M72/AS01E was evaluated in healthy, HIV-negative adolescents in a TB endemic region, regardless of Mycobacterium tuberculosis (M.tb) infection status.
Methods: In a phase II, double-blind randomized, controlled study (NCT00950612), two doses of M72/AS01E or placebo were administered intramuscularly, one month apart. Participants were followed-up post-vaccination, for 6 months. M72-specific immunogenicity was evaluated by intracellular cytokine staining analysis of T cells and NK cells by flow cytometry.
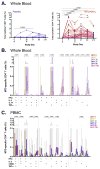
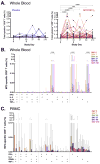
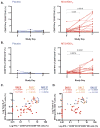
Results: No serious adverse events were recorded. M72/AS01E induced robust T cell and antibody responses, including antigen-dependent NK cell IFN-γ production. CD4 and CD8 T cell responses were sustained at 6 months post vaccination. Irrespective of M.tb infection status, vaccination induced a high frequency of M72-specific CD4 T cells expressing multiple combinations of Th1 cytokines, and low level IL-17. We observed rapid boosting of immune responses in M.tb-infected participants, suggesting natural infection acts as a prime to vaccination.
Conclusions: The clinically acceptable safety and immunogenicity profile of M72/AS01E in adolescents living in an area with high TB burden support the move to efficacy trials.
Keywords: Cytokine; M72/AS01(E); NK cell; T cell; Tuberculosis; Vaccine.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
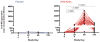

References
-
- World Health Organization. Global tuberculosis report 2014. Geneva: World Health Organization; 2014.
-
- Soysal A, Millington KA, Bakir M, Dosanjh D, Aslan Y, Deeks JJ, et al. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet. 2005;366:1443–51. - PubMed
-
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. J Am Med Assoc. 1994;271:698–702. - PubMed
-
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–80. - PubMed
-
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

