Direct wavefront sensing for high-resolution in vivo imaging in scattering tissue
- PMID: 26073070
- PMCID: PMC4490402
- DOI: 10.1038/ncomms8276
Direct wavefront sensing for high-resolution in vivo imaging in scattering tissue
Abstract
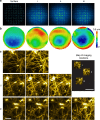
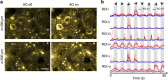
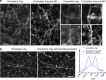
Adaptive optics by direct imaging of the wavefront distortions of a laser-induced guide star has long been used in astronomy, and more recently in microscopy to compensate for aberrations in transparent specimens. Here we extend this approach to tissues that strongly scatter visible light by exploiting the reduced scattering of near-infrared guide stars. The method enables in vivo two-photon morphological and functional imaging down to 700 μm inside the mouse brain.
Figures

References
-
- Helmchen F. & Denk W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005). - PubMed
-
- Kubby J. A. Adaptive Optics for Biological Imaging Taylor & Francis (2013).
-
- Kobat D. et al. Deep tissue multiphoton microscopy using longer wavelength excitation. Opt. Express 17, 13354–13364 (2009). - PubMed
-
- Ji N., Milkie D. E. & Betzig E. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nat. Methods 7, 141–147 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

