The SWI/SNF Subunit INI1 Contains an N-Terminal Winged Helix DNA Binding Domain that Is a Target for Mutations in Schwannomatosis
- PMID: 26073604
- PMCID: PMC4509781
- DOI: 10.1016/j.str.2015.04.021
The SWI/SNF Subunit INI1 Contains an N-Terminal Winged Helix DNA Binding Domain that Is a Target for Mutations in Schwannomatosis
Abstract
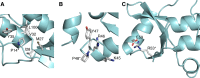
SWI/SNF complexes use the energy of ATP hydrolysis to remodel chromatin. In mammals they play a central role in regulating gene expression during differentiation and proliferation. Mutations in SWI/SNF subunits are among the most frequent gene alterations in cancer. The INI1/hSNF5/SMARCB1 subunit is mutated in both malignant rhabdoid tumor, a highly aggressive childhood cancer, and schwannomatosis, a tumor-predisposing syndrome characterized by mostly benign tumors of the CNS. Here, we show that mutations in INI1 that cause schwannomatosis target a hitherto unidentified N-terminal winged helix DNA binding domain that is also present in the BAF45a/PHF10 subunit of the SWI/SNF complex. The domain is structurally related to the SKI/SNO/DAC domain, which is found in a number of metazoan chromatin-associated proteins.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Bacci C., Sestini R., Provenzano A., Paganini I., Mancini I., Porfirio B., Vivarelli R., Genuardi M., Papi L. Schwannomatosis associated with multiple meningiomas due to a familial SMARCB1 mutation. Neurogenetics. 2010;11:73–80. - PubMed
-
- Boyd C., Smith M.J., Kluwe L., Balogh A., Maccollin M., Plotkin S.R. Alterations in the SMARCB1 (INI1) tumor suppressor gene in familial schwannomatosis. Clin. Genet. 2008;74:358–366. - PubMed
-
- Brunger A.T. Version 1.2 of the crystallography and NMR system. Nat. Protoc. 2007;2:2728–2733. - PubMed
-
- Cheng S.W., Davies K.P., Yung E., Beltran R.J., Yu J., Kalpana G.V. c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function. Nat. Genet. 1999;22:102–105. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

