Severe dermatitis, multiple allergies, and metabolic wasting syndrome caused by a novel mutation in the N-terminal plakin domain of desmoplakin
- PMID: 26073755
- PMCID: PMC4649901
- DOI: 10.1016/j.jaci.2015.05.002
Severe dermatitis, multiple allergies, and metabolic wasting syndrome caused by a novel mutation in the N-terminal plakin domain of desmoplakin
Abstract
Background: Severe dermatitis, multiple allergies, and metabolic wasting (SAM) syndrome is a recently recognized syndrome caused by mutations in the desmoglein 1 gene (DSG1). To date, only 3 families have been reported.
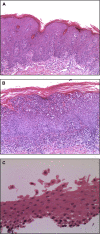
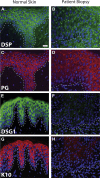
Objective: We studied a new case of SAM syndrome known to have no mutations in DSG1 to detail the clinical, histopathologic, immunofluorescent, and ultrastructural phenotype and to identify the underlying molecular mechanisms in this rare genodermatosis.
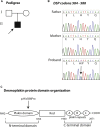
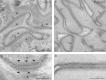
Methods: Histopathologic, electron microscopy, and immunofluorescent studies were performed. Whole-exome sequencing data were interrogated for mutations in desmosomal and other skin structural genes, followed by Sanger sequencing of candidate genes in the patient and his parents.
Results: No mutations were identified in DSG1; however, a novel de novo heterozygous missense c.1757A>C mutation in the desmoplakin gene (DSP) was identified in the patient, predicting the amino acid substitution p.His586Pro in the desmoplakin polypeptide.
Conclusions: SAM syndrome can be caused by mutations in both DSG1 and DSP. Knowledge of this genetic heterogeneity is important for both analysis of patients and genetic counseling of families. This condition and these observations reinforce the importance of heritable skin barrier defects, in this case desmosomal proteins, in the pathogenesis of atopic disease.
Keywords: Atopy; atopic dermatitis; atopic sensitization; desmoplakin; desmosome; eosinophilic esophagitis; skin barrier.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Chavanas S., Bodemer C., Rochat A., Hamel-Teillac D., Ali M., Irvine A.D. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–142. - PubMed
-
- Smith F.J., Irvine A.D., Terron-Kwiatkowski A., Sandilands A., Campbell L.E., Zhao Y. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–342. - PubMed
-
- Palmer C.N., Irvine A.D., Terron-Kwiatkowski A., Zhao Y., Liao H., Lee S.P. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. - PubMed
-
- Sandilands A., Smith F.J., Irvine A.D., McLean W.H. Filaggrin's fuller figure: a glimpse into the genetic architecture of atopic dermatitis. J Invest Dermatol. 2007;127:1282–1284. - PubMed
-
- Israeli S., Zamir H., Sarig O., Bergman R., Sprecher E. Inflammatory peeling skin syndrome caused by a mutation in CDSN encoding corneodesmosin. J Invest Dermatol. 2011;131:779–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 090066/B/09/Z/WT_/Wellcome Trust/United Kingdom
- MR/K001744/1/MRC_/Medical Research Council/United Kingdom
- WT083481/WT_/Wellcome Trust/United Kingdom
- R01 AR061106/AR/NIAMS NIH HHS/United States
- G0900740/MRC_/Medical Research Council/United Kingdom
- G0700314/MRC_/Medical Research Council/United Kingdom
- R01 AR041836/AR/NIAMS NIH HHS/United States
- WT092340/WT_/Wellcome Trust/United Kingdom
- 098439/Z/12/Z/WT_/Wellcome Trust/United Kingdom
- R01AR061106/AR/NIAMS NIH HHS/United States
- 098439/WT_/Wellcome Trust/United Kingdom
- WT097945/WT_/Wellcome Trust/United Kingdom
- R37 AR043380/AR/NIAMS NIH HHS/United States
- 092530/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- G0802780/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

