Comparing the efficacy of a web-assisted calprotectin-based treatment algorithm (IBD-live) with usual practices in teenagers with inflammatory bowel disease: study protocol for a randomized controlled trial
- PMID: 26073770
- PMCID: PMC4486429
- DOI: 10.1186/s13063-015-0787-x
Comparing the efficacy of a web-assisted calprotectin-based treatment algorithm (IBD-live) with usual practices in teenagers with inflammatory bowel disease: study protocol for a randomized controlled trial
Abstract
Background: To prevent clinical relapse in teenagers with inflammatory bowel disease (IBD) there is a need to monitor disease activity continuously. Timely optimisation of medical treatment may nip a preclinical relapse in the bud and change the natural course of IBD. Traditionally, disease monitoring is done during scheduled visits, but this is when most teenagers report full control. IBD care could be more efficient if patients were seen at times of clinical need. This study aims to examine the effectiveness of a web-assisted calprotectin-based treatment algorithm (IBD-live) compared with usual practices in teenagers with IBD.
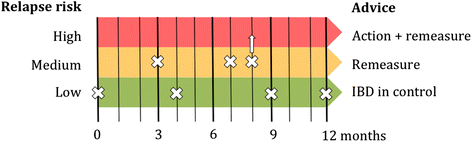
Methods/design: A randomized trial of web-based disease monitoring versus usual care is conducted at 10 Dutch IBD care centers. We plan to recruit 180 patients between 10- and 19-years old with quiescent IBD at baseline. Teenagers assigned to IBD-live will use the flarometer--an automatic cumulation of disease activity and fecal calprotectin measurements- to estimate probability of relapse. In case the flarometer indicates high risk the patient requires treatment intensification in accordance with national guidelines; low risk means that maintenance therapy is unchanged; and intermediate risk requires optimisation of drug adherence. Patients assigned to usual practice get the best accepted medical care with regular health checks. Primary outcome is the frequency of relapse at 52 weeks of follow-up. The diagnosis of relapse is based on a clinical activity index score >10 points necessitating remission induction therapy. Secondary outcomes include quality of life and cost-effectiveness.
Discussion: Web-assisted monitoring of disease activity with rapid access for those with acute relapse may allow teenagers to develop skills that are required of adult patients (including communication and self-determination). Similar monitoring systems have been introduced for teenagers with asthma and diabetes, with a positive effect on disease control, but the intervention has not been evaluated in teenagers with IBD. A randomized trial in adult patients with ulcerative colitis showed that a web-assisted treatment algorithm is feasible, safe and cost-effective. Results of the current trial are expected to have important implications for teenagers with IBD that incurs substantial health burdens and economic costs.
Trial registration: Dutch Trial Register identifier: NTR3759 (registered 29 December 2012).
Figures


References
-
- Romberg-Camps M, Hesselink-van de Kruijs M, Schouten L, Dagnelie P, Limonard C, Kester A, et al. Inflammatory bowel disease in South Limburg (the Netherlands) 1991–2002: incidence, diagnostic delay, and seasonal variations in onset of symptoms. J Crohn’s Colitis. 2009;3:115–24. doi: 10.1016/j.crohns.2008.12.002. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

