Loss-of-Function Mutations in APPL1 in Familial Diabetes Mellitus
- PMID: 26073777
- PMCID: PMC4571002
- DOI: 10.1016/j.ajhg.2015.05.011
Loss-of-Function Mutations in APPL1 in Familial Diabetes Mellitus
Abstract
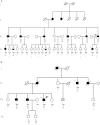
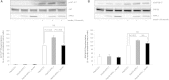
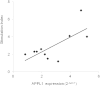
Diabetes mellitus is a highly heterogeneous disorder encompassing several distinct forms with different clinical manifestations including a wide spectrum of age at onset. Despite many advances, the causal genetic defect remains unknown for many subtypes of the disease, including some of those forms with an apparent Mendelian mode of inheritance. Here we report two loss-of-function mutations (c.1655T>A [p.Leu552(∗)] and c.280G>A [p.Asp94Asn]) in the gene for the Adaptor Protein, Phosphotyrosine Interaction, PH domain, and leucine zipper containing 1 (APPL1) that were identified by means of whole-exome sequencing in two large families with a high prevalence of diabetes not due to mutations in known genes involved in maturity onset diabetes of the young (MODY). APPL1 binds to AKT2, a key molecule in the insulin signaling pathway, thereby enhancing insulin-induced AKT2 activation and downstream signaling leading to insulin action and secretion. Both mutations cause APPL1 loss of function. The p.Leu552(∗) alteration totally abolishes APPL1 protein expression in HepG2 transfected cells and the p.Asp94Asn alteration causes significant reduction in the enhancement of the insulin-stimulated AKT2 and GSK3β phosphorylation that is observed after wild-type APPL1 transfection. These findings-linking APPL1 mutations to familial forms of diabetes-reaffirm the critical role of APPL1 in glucose homeostasis.
Copyright © 2015 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Roglic G., Unwin N., Bennett P.H., Mathers C., Tuomilehto J., Nag S., Connolly V., King H. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005;28:2130–2135. - PubMed
-
- Vaxillaire M., Froguel P. Monogenic diabetes in the young, pharmacogenetics and relevance to multifactorial forms of type 2 diabetes. Endocr. Rev. 2008;29:254–264. - PubMed
-
- Bamshad M.J., Ng S.B., Bigham A.W., Tabor H.K., Emond M.J., Nickerson D.A., Shendure J. Exome sequencing as a tool for Mendelian disease gene discovery. Nat. Rev. Genet. 2011;12:745–755. - PubMed
-
- Doria A., Yang Y., Malecki M., Scotti S., Dreyfus J., O’Keeffe C., Orban T., Warram J.H., Krolewski A.S. Phenotypic characteristics of early-onset autosomal-dominant type 2 diabetes unlinked to known maturity-onset diabetes of the young (MODY) genes. Diabetes Care. 1999;22:253–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

