Electron Tomography Analysis of Tick-Borne Encephalitis Virus Infection in Human Neurons
- PMID: 26073783
- PMCID: PMC4466586
- DOI: 10.1038/srep10745
Electron Tomography Analysis of Tick-Borne Encephalitis Virus Infection in Human Neurons
Abstract
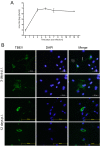
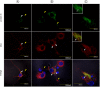
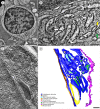
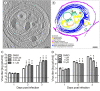
Tick-borne encephalitis virus (TBEV) causes serious, potentially fatal neurological infections that affect humans in endemic regions of Europe and Asia. Neurons are the primary target for TBEV infection in the central nervous system. However, knowledge about this viral infection and virus-induced neuronal injury is fragmental. Here, we directly examined the pathology that occurs after TBEV infection in human primary neurons. We exploited the advantages of advanced high-pressure freezing and freeze-substitution techniques to achieve optimal preservation of infected cell architecture. Electron tomographic (ET) reconstructions elucidated high-resolution 3D images of the proliferating endoplasmic reticulum, and individual tubule-like structures of different diameters in the endoplasmic reticulum cisternae of single cells. ET revealed direct connections between the tubule-like structures and viral particles in the endoplasmic reticulum. Furthermore, ET showed connections between cellular microtubules and vacuoles that harbored the TBEV virions in neuronal extensions. This study was the first to characterize the 3D topographical organization of membranous whorls and autophagic vacuoles in TBEV-infected human neurons. The functional importance of autophagy during TBEV replication was studied in human neuroblastoma cells; stimulation of autophagy resulted in significantly increased dose-dependent TBEV production, whereas the inhibition of autophagy showed a profound, dose-dependent decrease of the yield of infectious virus.
Figures




References
-
- Mansfield K. L. et al. Tick-borne encephalitis virus - a review of an emerging zoonosis. J. Gen. Virol. 90, 1781–94 (2009). - PubMed
-
- Růžek D., Dobler G., & Donoso Mantke O. Tick-borne encephalitis: pathogenesis and clinical implications. Travel Med. Infect. Dis. 8, 223–32 (2010). - PubMed
-
- Gelpi E. et al. Visualization of Central European tick-borne encephalitis infection in fatal human cases. J. Neuropathol. Exp. Neurol. 64, 506–12 (2005). - PubMed
-
- Gelpi E. et al. Inflammatory response in human tick-borne encephalitis: analysis of postmortem brain tissue. J. Neurovirol. 12, 322–7 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

