Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer
- PMID: 26073886
- PMCID: PMC4817877
- DOI: 10.1038/mt.2015.109
Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer
Abstract
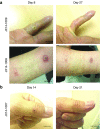
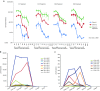
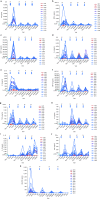
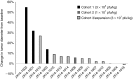
Fifteen patients with treatment-refractory colorectal cancer were enrolled on a phase 1b study of Pexa-Vec (pexastimogene devacirepvec; JX-594), an oncolytic and immunotherapeutic vaccinia designed to selectively replicate in cancer cells. Pexa-Vec was administered intravenously every 14 days, at dose levels of 1 × 10(6), 1 × 10(7), or 3 × 10(7) plaque-forming units (pfu)/kg. The primary endpoint was to determine the maximum tolerated dose. Secondary endpoints were pharmacokinetics and pharmacodynamics as well as antitumor activity. Patients were heavily pretreated (mean 4.5 lines of therapy). All patients received at least two Pexa-Vec doses (median = 4; range = 2-4). No dose-limiting toxicities were reported, and the maximum tolerated dose was not reached. The most common adverse events were grade 1/2 flu-like symptoms, generally lasting <24 hours. During the first and last cycles, genome pharmacokinetics were unchanged. Infectious pfu could be detected in plasma up to 2 hours after cycle 1 and up to 30 minutes after cycle 4 (when antivaccinia antibody titers are known to have peaked). Ten patients (67%) had radiographically stable disease. Given the acceptable safety profile of multiple intravenous Pexa-Vec infusions in patients with treatment-refractory colorectal cancer, further trials evaluating efficacy of intravenous Pexa-Vec, as monotherapy or in combination with chemotherapeutic agents, is warranted in this patient population.
Figures
Similar articles
-
Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial.Lancet Oncol. 2008 Jun;9(6):533-42. doi: 10.1016/S1470-2045(08)70107-4. Epub 2008 May 19. Lancet Oncol. 2008. PMID: 18495536 Clinical Trial.
-
Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients.Mol Ther. 2015 Mar;23(3):602-8. doi: 10.1038/mt.2014.243. Epub 2014 Dec 22. Mol Ther. 2015. PMID: 25531693 Free PMC article. Clinical Trial.
-
Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans.Sci Transl Med. 2013 May 15;5(185):185ra63. doi: 10.1126/scitranslmed.3005361. Sci Transl Med. 2013. PMID: 23677592 Clinical Trial.
-
The emerging therapeutic potential of the oncolytic immunotherapeutic Pexa-Vec (JX-594).Oncolytic Virother. 2015 Jan 28;4:25-31. doi: 10.2147/OV.S59640. eCollection 2015. Oncolytic Virother. 2015. PMID: 27512667 Free PMC article. Review.
-
Oncolytic virus therapy: A new era of cancer treatment at dawn.Cancer Sci. 2016 Oct;107(10):1373-1379. doi: 10.1111/cas.13027. Epub 2016 Sep 9. Cancer Sci. 2016. PMID: 27486853 Free PMC article. Review.
Cited by
-
Potentiating effect of reovirus on immune checkpoint inhibition in microsatellite stable colorectal cancer.Front Oncol. 2022 Oct 25;12:1018767. doi: 10.3389/fonc.2022.1018767. eCollection 2022. Front Oncol. 2022. PMID: 36387154 Free PMC article.
-
Remodeling of the tumor microenvironment using an engineered oncolytic vaccinia virus improves PD-L1 inhibition outcomes.Biosci Rep. 2021 Jun 25;41(6):BSR20204186. doi: 10.1042/BSR20204186. Biosci Rep. 2021. PMID: 34060602 Free PMC article.
-
Gemcitabine combined with an engineered oncolytic vaccinia virus exhibits a synergistic suppressive effect on the tumor growth of pancreatic cancer.Oncol Rep. 2019 Jan;41(1):67-76. doi: 10.3892/or.2018.6817. Epub 2018 Oct 24. Oncol Rep. 2019. PMID: 30365143 Free PMC article.
-
The Oncolytic Virus in Cancer Diagnosis and Treatment.Front Oncol. 2020 Sep 9;10:1786. doi: 10.3389/fonc.2020.01786. eCollection 2020. Front Oncol. 2020. Retraction in: Front Oncol. 2023 Nov 10;13:1320477. doi: 10.3389/fonc.2023.1320477. PMID: 33014876 Free PMC article. Retracted. Review.
-
Phase 2 trial of intravenous oncolytic virus JX-594 combined with low-dose cyclophosphamide in patients with advanced breast cancer.Exp Hematol Oncol. 2022 Dec 6;11(1):104. doi: 10.1186/s40164-022-00338-2. Exp Hematol Oncol. 2022. PMID: 36474303 Free PMC article.
References
-
- Douillard, JY, Siena, S, Cassidy, J, Tabernero, J, Burkes, R, Barugel, M et al. (2010). Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28: 4697–4705. - PubMed
-
- Peeters, M, Price, TJ, Cervantes, A, Sobrero, AF, Ducreux, M, Hotko, Y et al. (2010). Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28: 4706–4713. - PubMed
-
- Lièvre, A, Bachet, JB, Le Corre, D, Boige, V, Landi, B, Emile, JF et al. (2006). KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66: 3992–3995. - PubMed
-
- Grothey, A, Van Cutsem, E, Sobrero, A, Siena, S, Falcone, A, Ychou, M et al.; CORRECT Study Group. (2013). Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381: 303–312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

