A bi-functional antibody-receptor domain fusion protein simultaneously targeting IGF-IR and VEGF for degradation
- PMID: 26073904
- PMCID: PMC4623440
- DOI: 10.1080/19420862.2015.1055442
A bi-functional antibody-receptor domain fusion protein simultaneously targeting IGF-IR and VEGF for degradation
Abstract
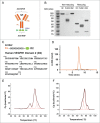
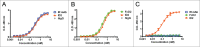
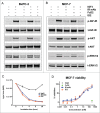
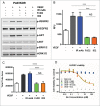
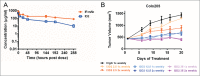
Bi-specific antibodies (BsAbs), which can simultaneously block 2 tumor targets, have emerged as promising therapeutic alternatives to combinations of individual monoclonal antibodies. Here, we describe the engineering and development of a novel, human bi-functional antibody-receptor domain fusion molecule with ligand capture (bi-AbCap) through the fusion of the domain 2 of human vascular endothelial growth factor receptor 1 (VEGFR1) to an antibody directed against insulin-like growth factor - type I receptor (IGF-IR). The bi-AbCap possesses excellent stability and developability, and is the result of minimal engineering. Beyond potent neutralizing activities against IGF-IR and VEGF, the bi-AbCap is capable of cross-linking VEGF to IGF-IR, leading to co-internalization and degradation of both targets by tumor cells. In multiple mouse xenograft tumor models, the bi-AbCap improves anti-tumor activity over individual monotherapies. More importantly, it exhibits superior inhibition of tumor growth, compared with the combination of anti-IGF-IR and anti-VEGF therapies, via powerful blockade of both direct tumor cell growth and tumor angiogenesis. The unique "capture-for-degradation" mechanism of the bi-AbCap is informative for the design of next-generation bi-functional anti-cancer therapies directed against independent signaling pathways. The bi-AbCap design represents an alternative approach to the creation of dual-targeting antibody fusion molecules by taking advantage of natural receptor-ligand interactions.
Keywords: IGF-IR; VEGF; VEGFR1; VEGFR2; angiogenesis; antibody fusion; bi-functional antibody; bispecific antibody; degradation; internalization.
Figures



References
-
- Chames P, Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? mAbs 2009; 1:539-47; PMID:20073127; http://dx.doi.org/10.4161/mabs.1.6.10015 - DOI - PMC - PubMed
-
- Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Dis Today 2015; PMID:25728220 - PubMed
-
- Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. mAbs 2010; 2:129-36; PMID:20190561; http://dx.doi.org/10.4161/mabs.2.2.11221 - DOI - PMC - PubMed
-
- Dong J, Demarest SJ, Sereno A, Tamraz S, Langley E, Doern A, Snipas T, Perron K, Joseph I, Glaser SM, et al.. Combination of two insulin-like growth factor-I receptor inhibitory antibodies targeting distinct epitopes leads to an enhanced antitumor response. Mol Cancer Ther 2010; 9:2593-604; PMID:20716637; http://dx.doi.org/10.1158/1535-7163.MCT-09-1018 - DOI - PubMed
-
- Lu D, Zhang H, Ludwig D, Persaud A, Jimenez X, Burtrum D, Balderes P, Liu M, Bohlen P, Witte L, et al.. Simultaneous blockade of both the epidermal growth factor receptor and the insulin-like growth factor receptor signaling pathways in cancer cells with a fully human recombinant bispecific antibody. J Biol Chem 2004; 279:2856-65; PMID:14576153; http://dx.doi.org/10.1074/jbc.M310132200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
