Iron Toxicity in the Retina Requires Alu RNA and the NLRP3 Inflammasome
- PMID: 26074074
- PMCID: PMC4481133
- DOI: 10.1016/j.celrep.2015.05.023
Iron Toxicity in the Retina Requires Alu RNA and the NLRP3 Inflammasome
Abstract
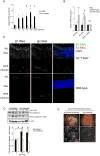
Excess iron induces tissue damage and is implicated in age-related macular degeneration (AMD). Iron toxicity is widely attributed to hydroxyl radical formation through Fenton's reaction. We report that excess iron, but not other Fenton catalytic metals, induces activation of the NLRP3 inflammasome, a pathway also implicated in AMD. Additionally, iron-induced degeneration of the retinal pigmented epithelium (RPE) is suppressed in mice lacking inflammasome components caspase-1/11 or Nlrp3 or by inhibition of caspase-1. Iron overload increases abundance of RNAs transcribed from short interspersed nuclear elements (SINEs): Alu RNAs and the rodent equivalent B1 and B2 RNAs, which are inflammasome agonists. Targeting Alu or B2 RNA prevents iron-induced inflammasome activation and RPE degeneration. Iron-induced SINE RNA accumulation is due to suppression of DICER1 via sequestration of the co-factor poly(C)-binding protein 2 (PCBP2). These findings reveal an unexpected mechanism of iron toxicity, with implications for AMD and neurodegenerative diseases associated with excess iron.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Blyn LB, Swiderek KM, Richards O, Stahl DC, Semler BL, Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11115–11120. - PMC - PubMed
-
- Chan C, Shen D, Wang Y, Chu X, Abu-Asab M, Tuo J. Inflammasomes in human eyes with AMD and mouse retinas with focal retinal degeneration; ARVO Annual Meeting; Seattle, WA. 2013. p. 315.
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000117/TR/NCATS NIH HHS/United States
- K99 EY024336/EY/NEI NIH HHS/United States
- UL1 RR033173/RR/NCRR NIH HHS/United States
- K99EY024336/EY/NEI NIH HHS/United States
- T32HL091812/HL/NHLBI NIH HHS/United States
- R01 EY022238/EY/NEI NIH HHS/United States
- R01EY018836/EY/NEI NIH HHS/United States
- R01EY020672/EY/NEI NIH HHS/United States
- UL1RR033173/RR/NCRR NIH HHS/United States
- DP1GM114862/DP/NCCDPHP CDC HHS/United States
- R00 EY024336/EY/NEI NIH HHS/United States
- R01EY022238/EY/NEI NIH HHS/United States
- R01 EY018350/EY/NEI NIH HHS/United States
- R01EY024068/EY/NEI NIH HHS/United States
- R01EY018350/EY/NEI NIH HHS/United States
- R01 EY018836/EY/NEI NIH HHS/United States
- R01 EY020672/EY/NEI NIH HHS/United States
- R01 EY015240/EY/NEI NIH HHS/United States
- T32 HL091812/HL/NHLBI NIH HHS/United States
- R01 EY024068/EY/NEI NIH HHS/United States
- K08 EY021757/EY/NEI NIH HHS/United States
- DP1 GM114862/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

